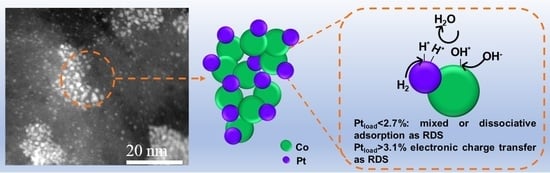
Ultra-Low Pt Loading in PtCo Catalysts for the Hydrogen Oxidation Reaction: What Role Do Co Nanoparticles Play?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of the Materials
2.2.1. Synthesis of the Support (MWCNT and rGO)
2.2.2. Synthesis of the Pt-Based Catalyst (Pt/MWCNT, Pt/rGO, and Pt/C)
2.2.3. Synthesis of the Template Co/MWCNT
2.2.4. Synthesis of the Catalysts PtCo/MWCNT
2.3. Physicochemical Characterization
2.4. Electrochemical Evaluation of the Catalysts
2.4.1. Electrochemical Cell and Instrument for the Determination of the Catalytic Activity
2.4.2. Estimation of the Electrochemically Surface Area
3. Results and Discussion
3.1. Physicochemical Characterization
3.2. Active Surface Area (ECSA) and the Oxophylic Nature of PtCo Catalysts
3.3. HOR Catalytic Activity and Mechanism
3.4. Stability of the Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akinyele, D.; Rayudu, R. Review of energy storage technologies for sustainable power networks. Sustain. Energy Technol. Assess. 2014, 8, 74–91. [Google Scholar] [CrossRef]
- Twidell, J.; Weir, A. Renewable Energy Resources, 2nd ed.; Routledge: London, UK, 2006. [Google Scholar]
- Loudiyi, K.; Berrada, A. Experimental Validation of Gravity Energy Storage Hydraulic Modeling. Energy Procedia 2017, 134, 845–854. [Google Scholar] [CrossRef]
- Zhan, J.; Ansari, O.A.; Liu, W.; Chung, C. An accurate bilinear cavern model for compressed air energy storage. Appl. Energy 2019, 242, 752–768. [Google Scholar] [CrossRef] [Green Version]
- Roberts, M.B.; Bruce, A.; MacGill, I. Impact of shared battery energy storage systems on photovoltaic self-consumption and electricity bills in apartment buildings. Appl. Energy 2019, 245, 78–95. [Google Scholar] [CrossRef]
- AlRafea, K.; Fowler, M.; Elkamel, A.; Hajimiragha, A. Integration of renewable energy sources into combined cycle power plants through electrolysis generated hydrogen in a new designed energy hub. Int. J. Hydrog. Energy 2016, 41, 16718–16728. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of cell performance in anion exchange membrane fuel cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Toghyani, S.; Fakhradini, S.; Afshari, E.; Baniasadi, E.; Jamalabadi, M.Y.A.; Shadloo, M.S. Optimization of operating parameters of a polymer exchange membrane electrolyzer. Int. J. Hydrog. Energy 2019, 44, 6403–6414. [Google Scholar] [CrossRef]
- Fang, R.; Liang, Y. Control strategy of electrolyzer in a wind-hydrogen system considering the constraints of switching times. Int. J. Hydrog. Energy 2019, 44, 25104–25111. [Google Scholar] [CrossRef]
- Cong, Y.; Yi, B.; Song, Y. Hydrogen oxidation reaction in alkaline media: From mechanism to recent electrocatalysts. Nano Energy 2018, 44, 288–303. [Google Scholar] [CrossRef]
- Dogdibegovic, E.; Wang, R.; Lau, G.Y.; Tucker, M. High performance metal-supported solid oxide fuel cells with infiltrated electrodes. J. Power Sources 2018, 410, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Tam, T.K.; Sun, F.; You, C.; Zhang, Y.H.P. A high-energy-density sugar biobattery based on a synthetic enzymatic pathway. Nat. Commun. 2014, 5, 3026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and fuels from electrochemical interfaces. Nat. Mater. 2016, 16, 57–69. [Google Scholar] [CrossRef]
- Zheng, X.; Shang, C.; Yang, J.; Wang, J.; Wang, L. Preparation and characterization of chitosan-crown ether membranes for alkaline fuel cells. Synth. Met. 2018, 247, 109–115. [Google Scholar] [CrossRef]
- Gasteiger, H.; Panels, J.; Yan, S. Dependence of PEM fuel cell performance on catalyst loading. J. Power Sources 2004, 127, 162–171. [Google Scholar] [CrossRef]
- Noerskov, J.K.; Bligaard, T.; Logadottir, A.; Kitchin, J.R.; Chen, J.G.; Pandelov, S.; Stimming, U. Trends in the Exchange Current for Hydrogen Evolution. J. Electrochem. Soc. 2005, 152, J23. [Google Scholar] [CrossRef] [Green Version]
- Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J.G.; Yan, Y. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Gasteiger, H.; Shao-Horn, Y. Hydrogen Oxidation and Evolution Reaction Kinetics on Platinum: Acid vs Alkaline Electrolytes. J. Electrochem. Soc. 2010, 157, B1529. [Google Scholar] [CrossRef]
- Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H.A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260. [Google Scholar] [CrossRef] [Green Version]
- Arenas, L.F.; Hadjigeorgiou, G.; Jones, S.; Van Dijk, N.; Hodgson, D.; Cruden, A.; de León, C.P. Effect of airbrush type on sprayed platinum and platinum-cobalt catalyst inks: Benchmarking as PEMFC and performance in an electrochemical hydrogen pump. Int. J. Hydrog. Energy 2020, 45, 27392–27403. [Google Scholar] [CrossRef]
- Zhuang, Z.; Giles, S.A.; Zheng, J.; Jenness, G.R.; Caratzoulas, S.; Vlachos, D.G.; Yan, Y. Nickel supported on nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. Nat. Commun. 2016, 7, 10141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, S.S.; Atkinson, R.W.; Unocic, R.; Zawodzinski, T.A.; Papandrew, A.B. Ruthenium-Alloy Electrocatalysts with Tunable Hydrogen Oxidation Kinetics in Alkaline Electrolyte. J. Phys. Chem. C 2015, 119, 13481–13487. [Google Scholar] [CrossRef]
- Miller, H.A.; Vizza, F.; Marelli, M.; Zadick, A.; Dubau, L.; Chatenet, M.; Geiger, S.; Cherevko, S.; Doan, H.; Pavlicek, R.; et al. Highly active nanostructured palladium-ceria electrocatalysts for the hydrogen oxidation reaction in alkaline medium. Nano Energy 2017, 33, 293–305. [Google Scholar] [CrossRef]
- Bakos, I.; Paszternák, A.; Zitoun, D. Pd/Ni Synergestic Activity for Hydrogen Oxidation Reaction in Alkaline Conditions. Electrochim. Acta 2015, 176, 1074–1082. [Google Scholar] [CrossRef] [Green Version]
- Alesker, M.; Page, M.; Shviro, M.; Paska, Y.; Gershinsky, G.; Dekel, D.R.; Zitoun, D. Palladium/nickel bifunctional electrocatalyst for hydrogen oxidation reaction in alkaline membrane fuel cell. J. Power Sources 2016, 304, 332–339. [Google Scholar] [CrossRef]
- Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Aguilar-Elguézabal, A.; Antúnez, W.; Alonso, G.; Delgado, F.P.; Espinosa, F.; Miki-Yoshida, M. Study of carbon nanotubes synthesis by spray pyrolysis and model of growth. Diam. Relat. Mater. 2006, 15, 1329–1335. [Google Scholar] [CrossRef]
- Chetty, R.; Kundu, S.; Xia, W.; Bron, M.; Schuhmann, W.; Chirila, V.; Brandl, W.; Reinecke, T.; Muhler, M. PtRu nanoparticles supported on nitrogen-doped multiwalled carbon nanotubes as catalyst for methanol electrooxidation. Electrochim. Acta 2009, 54, 4208–4215. [Google Scholar] [CrossRef]
- Li, W.; Liang, C.; Zhou, W.; Qiu, J.; Zhou, Z.; Sun, G.; Xin, Q. Preparation and Characterization of Multiwalled Carbon Nanotube-Supported Platinum for Cathode Catalysts of Direct Methanol Fuel Cells. J. Phys. Chem. B 2003, 107, 6292–6299. [Google Scholar] [CrossRef]
- Wang, D.; Yan, W.; Vijapur, S.; Botte, G.G. Electrochemically reduced graphene oxide–nickel nanocomposites for urea electrolysis. Electrochim. Acta 2013, 89, 732–736. [Google Scholar] [CrossRef]
- Beltrán-Gastélum, M.; Salazar-Gastélum, M.; Félix-Navarro, R.; Pérez-Sicairos, S.; Reynoso-Soto, E.; Lin, S.; Flores-Hernández, J.; Romero-Castañón, T.; Albarrán-Sánchez, I.; Paraguay-Delgado, F. Evaluation of Pt Au/MWCNT (Multiwalled Carbon Nanotubes) electrocatalyst performance as cathode of a proton exchange membrane fuel cell. Energy 2016, 109, 446–455. [Google Scholar] [CrossRef]
- Beltrán-Gastélum, M.; Salazar-Gastélum, M.; Flores-Hernández, J.; Botte, G.; Pérez-Sicairos, S.; Romero-Castañon, T.; Reynoso-Soto, E.; Félix-Navarro, R. Pt-Au nanoparticles on graphene for oxygen reduction reaction: Stability and performance on proton exchange membrane fuel cell. Energy 2019, 181, 1225–1234. [Google Scholar] [CrossRef]
- Alia, S.M.; Zhang, G.; Kisailus, D.; Li, D.; Gu, S.; Jensen, K.; Yan, Y. Porous Platinum Nanotubes for Oxygen Reduction and Methanol Oxidation Reactions. Adv. Funct. Mater. 2010, 20, 3742–3746. [Google Scholar] [CrossRef]
- Pollet, B.G. The use of ultrasound for the fabrication of fuel cell materials. Int. J. Hydrog. Energy 2010, 35, 11986–12004. [Google Scholar] [CrossRef]
- Zin, V.; Pollet, B.G.; Dabalà, M. Sonoelectrochemical (20 kHz) production of platinum nanoparticles from aqueous solutions. Electrochim. Acta 2009, 54, 7201–7206. [Google Scholar] [CrossRef]
- Scofield, M.E.; Zhou, Y.; Yue, S.; Wang, L.; Su, D.; Tong, X.; Vukmirovic, M.B.; Adzic, R.R.; Wong, S.S. Role of Chemical Composition in the Enhanced Catalytic Activity of Pt-Based Alloyed Ultrathin Nanowires for the Hydrogen Oxidation Reaction under Alkaline Conditions. ACS Catal. 2016, 6, 3895–3908. [Google Scholar] [CrossRef]
- Campos-Roldán, C.; Huerta, R.D.G.G.; Alonso-Vante, N. The oxophilic and electronic effects on anchored platinum nanoparticles on sp carbon sites: The hydrogen evolution and oxidation reactions in alkaline medium. Electrochim. Acta 2018, 283, 1829–1834. [Google Scholar] [CrossRef]
- Brukh, R.; Sae-Khow, O.; Mitra, S. Stabilizing single-walled carbon nanotubes by removal of residual metal catalysts. Chem. Phys. Lett. 2008, 459, 149–152. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Ayoko, G.A.; Bell, J.; Sun, Z. Cobalt oxide-based nanoarchitectures for electrochemical energy applications. Prog. Mater. Sci. 2019, 103, 596–677. [Google Scholar] [CrossRef]
- Benchettara, A.; Benchettara, A. Electrochemical Sensor Based on Nanoparticles of Cobalt Oxides for Determination of Glucose. Mater. Today Proc. 2015, 2, 4212–4216. [Google Scholar] [CrossRef]
- Oshchepkov, A.G.; Bonnefont, A.; Pronkin, S.; Cherstiouk, O.V.; Ulhaq-Bouillet, C.; Papaefthymiou, V.; Parmon, V.N.; Savinova, E.R. Nanostructured nickel nanoparticles supported on vulcan carbon as a highly active catalyst for the hydrogen oxidation reaction in alkaline media. J. Power Sources 2018, 402, 447–452. [Google Scholar] [CrossRef]
- Cruz-Reyes, I.; Trujillo-Navarrete, B.; García-Tapia, K.; Salazar-Gastélum, M.; Paraguay-Delgado, F.; Félix-Navarro, R. Pd/MnO2 as a bifunctional electrocatalyst for potential application in alkaline fuel cells. Fuel 2020, 279, 118470. [Google Scholar] [CrossRef]
- Long, C.; Wang, K.; Shi, Y.; Yang, Z.; Zhang, X.; Zhang, Y.; Han, J.; Bao, Y.; Chang, L.; Liu, S.; et al. Tuning the electronic structure of PtRu bimetallic nanoparticles for promoting the hydrogen oxidation reaction in alkaline media. Inorg. Chem. Front. 2019, 6, 2900–2905. [Google Scholar] [CrossRef]
- Yang, F.; Bao, X.; Zhao, Y.; Wang, X.; Cheng, G.; Luo, W. Enhanced HOR catalytic activity of PGM-free catalysts in alkaline media: The electronic effect induced by different heteroatom doped carbon supports. J. Mater. Chem. A 2019, 7, 10936–10941. [Google Scholar] [CrossRef]
- Duan, Y.; Yu, Z.-Y.; Yang, L.; Zheng, L.-R.; Zhang, C.-T.; Yang, X.-T.; Gao, F.-Y.; Zhang, X.-L.; Yu, X.; Liu, R.; et al. Bimetallic nickel-molybdenum/tungsten nanoalloys for high-efficiency hydrogen oxidation catalysis in alkaline electrolytes. Nat. Commun. 2020, 11, 4789. [Google Scholar] [CrossRef] [PubMed]






| Catalysts | †‡ Pt NPs Dp (nm) | †‡ Pt NPs Ap (nm2) | Pt NPs Ap/Co NPs A (%) |
|---|---|---|---|
| PtCo/MWCNT 1 | 1.41 ± 0.23 | 1.30 ± 0.51 | 6.2 |
| PtCo/MWCNT 2 | 1.35 ± 0.24 | 1.11 ± 0.41 | 6.6 |
| PtCo/MWCNT 3 | 1.44 ± 0.28 | 1.41 ± 0.62 | 16.1 |
| PtCo/MWCNT 4 | 1.53 ± 0.28 | 1.72 ± 0.65 | 20.2 |
| PtCo/MWCNT 6 | 1.65 ± 0.29 | 1.47 ± 0.60 | 25.4 |
| Catalysts | K2PtCl6 Solution Added (mL) | Pt Added (μmolPt) | Pt Concentration (μM) | %Pt 1 | %Co 1 |
|---|---|---|---|---|---|
| PtCo/MWCNT 1 | 1 | 1.026 | 20.513 | 0.438 | 13.117 |
| PtCo/MWCNT 2 | 2 | 2.051 | 41.026 | 1.568 | 12.176 |
| PtCo/MWCNT 3 | 3 | 3.077 | 61.538 | 2.709 | 12.065 |
| PtCo/MWCNT 4 | 4 | 4.102 | 82.051 | 3.108 | 11.718 |
| PtCo/MWCNT 6 | 6 | 6.154 | 123.077 | 4.034 | 10.134 |
| Catalysts | Ep (mV vs. Ag/AgCl) | Jp (mA cm−2) | Q (mC) | ECSA (m2 gr−1Pt) |
|---|---|---|---|---|
| Pt/C | 663 | 21.40 | 7.17 | 74.7 |
| Pt/MWCNT | 665 | 14.50 | 2.57 | 27.72 |
| Co/MWCNT | - | - | - | - |
| PtCo/MWCNT 1 | - | - | - | - |
| PtCo/MWCNT 2 | 750 | 2.74 | 0.43 | 56.70 |
| PtCo/MWCNT 3 | 720 | 8.16 | 0.83 | 63.56 |
| PtCo/MWCNT 4 | 685 | 6.51 | 0.88 | 59.17 |
| PtCo/MWCNT 6 | 680 | 9.70 | 1.44 | 74.48 |
| Catalysts | E1/2 (mV) | Jlim (mA cm−2) | K–L Slope (cm−2 mA−1 s−1/2) | Tafel Slope (mV dec−1) | A | J0 (mA cm−2) | Ref. |
|---|---|---|---|---|---|---|---|
| Pt/C | 76 | 5.00 | 1.22 | 123.9 | 0.21 | 1.36 | This work |
| Pt/MWCNT | 60 | 6.20 | 3.16 | 121.8 | 0.21 | 2.21 | This work |
| Pt/rGO | 165 | 0.65 | - | - | - | - | This work |
| Co/MWCNT | - | - | - | - | - | - | This work |
| PtCo/MWCNT 1 | - | - | - | - | - | - | This work |
| PtCo/MWCNT 2 | 510 | 1.72 | 3.41 | 73.4 | 0.35 | 0.02 | This work |
| PtCo/MWCNT 3 | 420 | 2.12 | 3.71 | 90.7 | 0.28 | 0.08 | This work |
| PtCo/MWCNT 4 | 364 | 2.44 | 3.16 | 121.3 | 0.21 | 0.20 | This work |
| PtCo/MWCNT 6 | 187 | 2.28 | 3.63 | 120.5 | 0.21 | 0.39 | This work |
| Pt(pc) | 65 | 2.70 | 4.87 | 125 | 0.50 | - | [18] |
| Ni/N-NTC | 25 | 1.30 | 5.21 | - | 0.45 | 0.03 3 | [21] |
| Ni/C | 28 | 1.60 1 | 3.50 | - | - | - | [41] |
| RuPt/C | 15 | 3.20 2 | - | 35 | - | 1.42 3 | [22] |
| RuPd/C | 95 | 3.30 3 | - | 219 | 0.27 | 0.15 3 | [22] |
| Pd/MnO2 | 279 | 2.33 | - | 35 | - | 10.4 3 | [42] |
| PtRu | 27 | 2.10 1 | - | - | - | 4.00 | [43] |
| Ni/SC | 25 | 1.42 1 | - | - | 0.54 | 0.04 3 | [44] |
| MoNi4 | 18 | 2.85 | 4.60 | 48.6 | - | 3.41 | [45] |
| WNi4 | 23 | 2.50 | 4.32 | - | - | 1.87 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anaya-Castro, F.d.J.; Beltrán-Gastélum, M.; Morales Soto, O.; Pérez-Sicairos, S.; Lin, S.W.; Trujillo-Navarrete, B.; Paraguay-Delgado, F.; Salazar-Gastélum, L.J.; Romero-Castañón, T.; Reynoso-Soto, E.; et al. Ultra-Low Pt Loading in PtCo Catalysts for the Hydrogen Oxidation Reaction: What Role Do Co Nanoparticles Play? Nanomaterials 2021, 11, 3156. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113156
Anaya-Castro FdJ, Beltrán-Gastélum M, Morales Soto O, Pérez-Sicairos S, Lin SW, Trujillo-Navarrete B, Paraguay-Delgado F, Salazar-Gastélum LJ, Romero-Castañón T, Reynoso-Soto E, et al. Ultra-Low Pt Loading in PtCo Catalysts for the Hydrogen Oxidation Reaction: What Role Do Co Nanoparticles Play? Nanomaterials. 2021; 11(11):3156. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113156
Chicago/Turabian StyleAnaya-Castro, Felipe de Jesús, Mara Beltrán-Gastélum, Omar Morales Soto, Sergio Pérez-Sicairos, Shui Wai Lin, Balter Trujillo-Navarrete, Francisco Paraguay-Delgado, Luis Javier Salazar-Gastélum, Tatiana Romero-Castañón, Edgar Reynoso-Soto, and et al. 2021. "Ultra-Low Pt Loading in PtCo Catalysts for the Hydrogen Oxidation Reaction: What Role Do Co Nanoparticles Play?" Nanomaterials 11, no. 11: 3156. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11113156