Facile Synthesis of Zn-Co-S Nanostrip Cluster Arrays on Ni Foam for High-Performance Hybrid Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
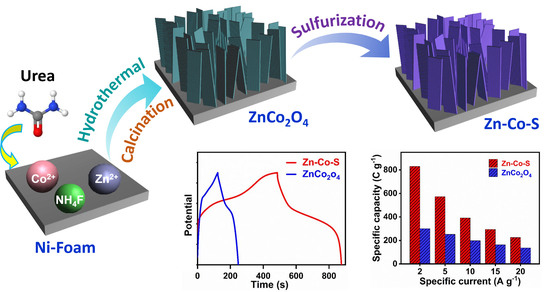
2.2. Synthesis of Zn-Co-S Nanostrip
2.3. Materials Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
3.1. Structural and Morphological Analysis
3.2. Electrochemical Performance of Zn-Co-S
3.3. Electrochemical Performance of Zn-Co-S//AC Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xie, M.; Duan, S.; Shen, Y.; Fang, K.; Wang, Y.; Lin, M.; Guo, X. In-situ-grown Mg(OH)2-derived hybrid α-Ni(OH)2 for highly stable supercapacitor. ACS Energy Lett. 2016, 1, 814–819. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Alfantazi, A.; Lee, Y.R.; Lee, M.; Shim, J.-J. Chemically synthesized copper sulfide nanoflakes on reduced graphene oxide for asymmetric supercapacitors. J. Ind. Eng. Chem. 2021, 101, 423–429. [Google Scholar] [CrossRef]
- Kumar, R.; Rai, P.; Sharma, A. Free-standing NiV2 S4 nanosheet arrays on a 3D Ni framework via an anion exchange reaction as a novel electrode for asymmetric supercapacitor applications. J. Mater. Chem. A 2016, 4, 17512–17520. [Google Scholar] [CrossRef]
- Ma, F.-X.; Yu, L.; Xu, C.-Y.; Lou, X.W.D. Self-supported formation of hierarchical NiCo2O4 tetragonal microtubes with enhanced electrochemical properties. Energy Environ. Sci. 2016, 9, 862–866. [Google Scholar] [CrossRef]
- Akinwolemiwa, B.; Peng, C.; Chen, G.Z. Redox electrolytes in supercapacitors. J. Electrochem. Soc. 2015, 162, A5054–A5059. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, G.; Mohapatra, D.; Tamang, T.L.; Lee, M.; Lee, Y.R.; Shim, J.-J. Redox-additive electrolyte–driven enhancement of the electrochemical energy storage performance of asymmetric Co3O4//carbon nano-onions supercapacitors. Energy 2021, 218, 119436–119447. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, D.; Chen, S.; Guan, Y.; Xiong, J. Construction of hierarchical NiCo2S4@ Ni(OH)2 core-shell hybrid nanosheet arrays on Ni foam for high-performance aqueous hybrid supercapacitors. Electrochim. Acta 2016, 193, 116–127. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Hussain, I.; Shim, J.-J. One-step synthesis of hollow C-NiCo2S4 nanostructures for high-performance supercapacitor electrodes. Nanoscale 2018, 10, 6620–6628. [Google Scholar] [CrossRef]
- Cao, W.; Liu, Y.; Xu, F.; Xia, Q.; Du, G.; Fan, Z.; Chen, N. Metal-organic framework derived carbon-coated spherical bimetallic nickel-cobalt sulfide nanoparticles for hybrid supercapacitors. Electrochim. Acta 2021, 385, 138433. [Google Scholar] [CrossRef]
- Guo, M.; Balamurugan, J.; Thanh, T.D.; Kim, N.H.; Lee, J.H. Facile fabrication of Co2CuS4 nanoparticle anchored N-doped graphene for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 17560–17571. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Shim, J.-J. In situ growth of hierarchical mesoporous NiCo2S4@MnO2 arrays on nickel foam for high-performance supercapacitors. Electrochim. Acta 2015, 166, 302–309. [Google Scholar] [CrossRef]
- Zhang, P.; Guan, B.Y.; Yu, L.; Lou, X.W. Formation of double-shelled zinc–cobalt sulfide dodecahedral cages from bimetallic zeolitic imidazolate frameworks for hybrid supercapacitors. Angew. Chem. 2017, 129, 7247–7251. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Sun, C.; Guo, G.; Sun, W.; Huang, W.; Yan, Q.; Dong, X. Controlled synthesis of zinc cobalt sulfide nanostructures in oil phase and their potential applications in electrochemical energy storage. J. Mater. Chem. A 2015, 3, 11462–11470. [Google Scholar] [CrossRef]
- Yed, J.A.; Ma, J.; Zhu, B.; Tang, S.; Meng, X. Hierarchical multicomponent electrode with interlaced Ni(OH)2 nanoflakes wrapped zinc cobalt sulfide nanotube arrays for sustainable high-performance supercapacitors. Adv. Energy Mater. 2017, 7, 1701228–1701240. [Google Scholar]
- Kuai, Y.; Liu, M.; Wang, T.; Fu, Y.; Ma, H.; Jiang, Q.; Guan, C.; Hu, K. Sea anemone-like zinc-cobalt oxysulfides grown on Ni foam as a battery-type electrode with admirable performance. Ionics 2017, 23, 1391–1398. [Google Scholar] [CrossRef]
- Xu, K.; Zou, R.; Li, W.; Liu, Q.; Liu, X.; An, L.; Hu, J. Design and synthesis of 3D interconnected mesoporous NiCo2O4@CoxNi 1− x (OH)2 core–shell nanosheet arrays with large areal capacitance and high rate performance for supercapacitors. J. Mater. Chem. A 2014, 2, 10090–10097. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, W.; Wang, G. The perfect matching between the low-cost Fe2O3 nanowire anode and the NiO nanoflake cathode significantly enhances the energy density of asymmetric supercapacitors. J. Mater. Chem. A 2015, 3, 6662–6670. [Google Scholar] [CrossRef]
- Sahoo, S.; Shim, J.-J. Nanostructured 3D zinc cobaltite/nitrogen-doped reduced graphene oxide composite electrode for supercapacitor applications. J. Ind. Eng. Chem. 2017, 54, 205–217. [Google Scholar] [CrossRef]
- Wu, C.; Cai, J.; Zhang, Q.; Zhou, X.; Zhu, Y.; Li, L.; Shen, P.; Zhang, K. Direct growth of urchin-like ZnCo2O4 microspheres assembled from nanowires on nickel foam as high-performance electrodes for supercapacitors. Electrochim. Acta 2015, 169, 202–209. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Lee, S.-H.; Ryu, K.-S. Synthesis of Zn3V2O8 nanoplatelets for lithium-ion battery and supercapacitor applications. RSC Adv. 2015, 5, 91822–91828. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, X.; Tong, L.; Luo, J.; Wang, Y.; Zhang, Y.; Xie, K.; Wu, Y. A facile synthesis of mesoporous Co3O4/CeO2 hybrid nanowire arrays for high performance supercapacitors. J. Mater. Chem. A 2015, 3, 10425–10431. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Han, X.; Cai, J.; Liu, M.; Yang, Y.; Zhang, K. 3D hierarchically porous zinc–nickel–cobalt oxide nanosheets grown on Ni foam as binder-free electrodes for electrochemical energy storage. J. Mater. Chem. A 2015, 3, 24022–24032. [Google Scholar] [CrossRef]
- Elshahawy, A.M.; Li, X.; Zhang, H.; Hu, Y.; Ho, K.H.; Guan, C.; Wang, J. Controllable MnCo2S4 nanostructures for high performance hybrid supercapacitors. J. Mater. Chem. A 2017, 5, 7494–7506. [Google Scholar] [CrossRef]
- Cao, L.; Tang, G.; Mei, J.; Liu, H. Construct hierarchical electrode with NixCo3-xS4 nanosheet coated on NiCo2O4 nanowire arrays grown on carbon fiber paper for high-performance asymmetric supercapacitors. J. Power Sources 2017, 359, 262–269. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, X.; Liu, Y.; He, Y.; Gao, Y.; Liu, J. High rate capabilities of NiCo2O4-based hierarchical superstructures for rechargeable charge storage. J. Electrochem. Soc. 2014, 161, A1922–A1926. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, F.; Nie, Z.; Qian, H.; Liu, P.; He, H.; Wu, J.; Chen, Z.; Chen, S. A high-performance battery-like supercapacitor electrode with a continuous NiTe network skeleton running throughout Co(OH)2/Co9S8 nanohybrid. Electrochim. Acta 2021, 365, 137325. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Muralidharan, G. Porous NiO/C nanocomposites as electrode material for electrochemical supercapacitors. ACS Sustain. Chem. Eng. 2013, 1, 1110–1118. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Nagamuthu, S.; Lee, S.-H.; Ryu, K.-S. Porous thin layered nanosheets assembled ZnCo2O4 grown on Ni-foam as an efficient electrode material for hybrid supercapacitor applications. Int. J. Hydrogen Energy 2017, 42, 3122–3129. [Google Scholar] [CrossRef]
- Cai, D.; Wang, D.; Wang, C.; Liu, B.; Wang, L.; Liu, Y.; Li, Q.; Wang, T. Construction of desirable NiCo2S4 nanotube arrays on nickel foam substrate for pseudocapacitors with enhanced performance. Electrochim. Acta 2015, 151, 35–41. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate methods for evaluating the efficiency and capacitive behavior of different types of supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Nagamuthu, S.; Vijayakumar, S.; Lee, S.-H.; Ryu, K.-S. Hybrid supercapacitor devices based on MnCo2O4 as the positive electrode and FeMn2O4 as the negative electrode. Appl. Surf. Sci. 2016, 390, 202–208. [Google Scholar] [CrossRef]
- Tong, H.; Bai, W.; Yue, S.; Gao, Z.; Lu, L.; Shen, L.; Dong, S.; Zhu, J.; He, J.; Zhang, X. Zinc cobalt sulfide nanosheets grown on nitrogen-doped graphene/carbon nanotube film as a high-performance electrode for supercapacitors. J. Mater. Chem. A 2016, 4, 11256–11263. [Google Scholar] [CrossRef]
- Hussain, I.; Lamiel, C.; Mohamed, S.G.; Vijayakumar, S.; Ali, A.; Shim, J.-J. Controlled synthesis and growth mechanism of zinc cobalt sulfide rods on Ni-foam for high-performance supercapacitors. J. Ind. Eng. Chem. 2019, 71, 250–259. [Google Scholar] [CrossRef]
- Wu, C.; Cai, J.; Zhang, Q.; Zhou, X.; Zhu, Y.; Shen, P.K.; Zhang, K. Hierarchical mesoporous Zinc–Nickel–Cobalt ternary oxide nanowire arrays on nickel foam as high-performance electrodes for supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 26512–26521. [Google Scholar] [CrossRef] [PubMed]
- Seol, M.L.; Nam, I.; Ribeiro, E.L.; Segel, B.; Lee, D.; Palma, T.; Wu, H.; Mukherjee, D.; Khomami, B.; Hill, C.; et al. All-Printed In-Plane Supercapacitors by Sequential Additive Manufacturing Process. ACS Appl. Energy Mater. 2020, 3, 4965–4973. [Google Scholar] [CrossRef]
- Hussain, I.; Mohapatra, D.; Dhakal, G.; Lamiel, C.; Mohamed, S.G.; Sayed, M.S.; Shim, J.-J. Different controlled nanostructures of Mn-doped ZnS for high-performance supercapacitor applications. J. Energy Storage 2020, 32, 101767. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Wang, J.; Han, P.; Xu, T.; Sun, Y.; Zhang, X.; Yang, H. Polyoxometalates@ metal-organic frameworks derived porous MoO3@ CuO as electrodes for symmetric all-solid-state supercapacitor. Electrochim. Acta 2016, 191, 795–804. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Fu, W.; Zhang, P.; Pan, X.; Xie, E. In situ synthesis of CoSx@carbon core-shell nanospheres decorated in carbon nanofibers for capacitor electrodes with superior rate and cycling performances. Carbon 2017, 114, 187–197. [Google Scholar] [CrossRef]
- Lei, K.; Ling, J.; Zhou, J.; Zou, H.; Yang, W.; Chen, S. Formation of CoS2/N, S-codoped porous carbon nanotube composites based on bimetallic zeolitic imidazolate organic frameworks for supercapacitors. Mater. Res. Bull. 2019, 116, 59–66. [Google Scholar] [CrossRef]
- Sahoo, A.; Sharma, Y. Synthesis and characterization of nanostructured ternary zinc manganese oxide as novel supercapacitor material. Mater. Chem. Phys. 2015, 149, 721–727. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vijayakumar, S.; Dhakal, G.; Kim, S.-H.; Lee, J.; Lee, Y.R.; Shim, J.-J. Facile Synthesis of Zn-Co-S Nanostrip Cluster Arrays on Ni Foam for High-Performance Hybrid Supercapacitors. Nanomaterials 2021, 11, 3209. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123209
Vijayakumar S, Dhakal G, Kim S-H, Lee J, Lee YR, Shim J-J. Facile Synthesis of Zn-Co-S Nanostrip Cluster Arrays on Ni Foam for High-Performance Hybrid Supercapacitors. Nanomaterials. 2021; 11(12):3209. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123209
Chicago/Turabian StyleVijayakumar, Subbukalai, Ganesh Dhakal, Soo-Hyun Kim, Jintae Lee, Yong Rok Lee, and Jae-Jin Shim. 2021. "Facile Synthesis of Zn-Co-S Nanostrip Cluster Arrays on Ni Foam for High-Performance Hybrid Supercapacitors" Nanomaterials 11, no. 12: 3209. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123209