A Pragmatic Perspective of the Antibacterial Properties of Metal-Based Nanoparticles
Abstract
:1. Introduction
2. Nanoparticle Surface Oxidation
3. The Antibacterial Properties of Metal Oxides
4. The Antibacterial Properties of Oxometallates
5. The Surface Structures of Metal Oxides
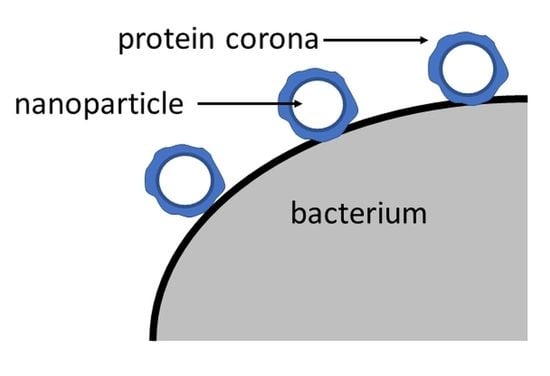
6. The Protein Corona
- The hard corona is generally irreversibly adsorbed.
- The proteins adsorbed depend on the nanoparticle material (Ag, Au, Fe3O4, etc.).
- For a given nanoparticle material, the proteins depend on nanoparticle shape.
- Gram-positive bacteria have a surface layer comprised of a thick transpeptidation-crosslinked [40] latticework of peptidoglycan.
- Gram-negative bacteria have a surface layer comprised of a lipid bilayer, whose outer leaf is composed of glycolipids, and whose inner leaf is composed of phospholipids.
7. The Exceptional Antibacterial Efficacy of Nanoparticle Alloys
8. Summary and Commercialization
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dollwet, H.H. Historic uses of Cu compounds in medicine. Trace Elem. Med. 1985, 2, 80–87. [Google Scholar]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snchez-Lpez, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Yahia, L.’H.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Cherry, B.W. Polymer Surfaces; Cambridge University Press: New York, NY, USA, 1981; Chapter 1. [Google Scholar]
- Bavand, R.; Chen, L.; França, R.; Loran, S.; Yang, D.Q.; Yelon, A.; Zhang, G.X.; Sacher, E. Comment on “Intensity modulation of the Shirley background of the Cr3p spectra with photon energies around the Cr2p edge”, by A Herrera-Gomez, D Cabrera-German, A D Dutol et al., Surface Interface Anal, 2018;50:246–252. Surf. Interface Anal. 2018, 50, 683–685. [Google Scholar] [CrossRef]
- Loran, S.; Cheng, S.; Botton, G.; Yahia, L.; Yelon, A.; Sacher, E. The physicochemical characterization of the Cu nanoparticle surface, and of its evolution on atmospheric exposure: Application to antimicrobial bandages for wound dressings. Appl. Surf. Sci. 2019, 473, 25–30. [Google Scholar] [CrossRef]
- Loran, S.; Yelon, A.; Sacher, E. Short communication: Unexpected findings on the physicochemical characterization of the silver nanoparticle surface. Appl. Surf. Sci. 2018, 428, 1179–1181. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, D.-Q.; Sacher, E. X-ray Photoelectron Spectroscopic Analysis of Pt Nanoparticles on Highly Oriented Pyrolytic Graphite, Using Symmetric Component Line Shapes. J. Phys. Chem. C 2007, 111, 565–570. [Google Scholar] [CrossRef]
- Poulin, S.; Ndzebet, E.; Debrie, A.; Savadogo, O.; Sacher, E. The cleaning and thiolation of commercial titanium for use in dental prostheses. Appl. Surf. Sci. 1999, 143, 238–244. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.; Khan, M.A.M.; Karuppiah, P.; Al-Dhabi, N.A. Synthesis, Characterization, and Antimicrobial Activity of Copper Oxide Nanoparticles. J. Nanomater. 2014, 2014, 637858. [Google Scholar] [CrossRef]
- Li, D.; Chen, S.; Zhang, K.; Gao, N.; Zhang, M.; Albasher, G.; Shi, J.; Wang, C. The interaction of Ag2O nanoparticles with Escherichia coli: Inhibition–sterilization process. Sci. Rep. 2021, 11, 1703. [Google Scholar] [CrossRef] [PubMed]
- Sacher, E. Asymmetries in Transition Metal XPS Spectra: Metal Nanoparticle Structure, and Interaction with the Graphene-Structured Substrate Surface. Langmuir 2010, 26, 3807–3814. [Google Scholar] [CrossRef]
- NIST XPS Database. Available online: https://srdata.nist.gov/XPS/ (accessed on 31 October 2021).
- Fukuda, N.; Yamase, T. In vitro antibacterial activity of vanadate and vanadyl compounds against Streptococcus pneumoniae. Biol. Pharm. Bull. 1997, 20, 927–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorani, G.; Modugno, G.; Bonchio, M.; Carraro, M. Nanosized metal oxides (NMOs) and polyoxometalates (POMs) for antibacterial water treatment. In Application of Nanotechnology in Membranes for Water Treatment; Figoli, A., Hoinkis, J., Altinkaya, S.A., Bundschuh, J., Eds.; CRC Press: London, UK, 2017; Chapter 9. [Google Scholar]
- Parkinson, G.S. Iron Oxide Surfaces. arXiv 2016, arXiv:1602.06774. [Google Scholar]
- Brown Gordon, E., Jr.; Henrich, V.; Casey, W.; Clark, D.; Eggleston, C.; Andrew Felmy, A.F.; Goodman, D.W.; Gratzel, M.; Maciel, G.; McCarthy, M.I.; et al. Metal Oxide Surfaces and Their Interactions with Aqueous Solutions and Microbial Organisms. Chem. Rev. 1999, 99, 77–174. [Google Scholar] [CrossRef] [Green Version]
- Kosmulski, M. Isoelectric points and points of zero charge of metal (hydr)oxides: 50 years after Parks’ review. Adv. Colloid Interface Sci. 2016, 238, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.R.; Feeney, R.E.; Sternberg, M.M. Chemical and physical modification of proteins by the hydroxyl ion. CRC Crit. Rev. Food Sci. Nutr. 1983, 19, 173–212. [Google Scholar] [CrossRef] [PubMed]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between Metal Oxides and Biomolecules: From Fundamental Understanding to Applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, J.; Radeke, C.; Feryab, A.; Chanana, M. The role of pH, metal ions and their hydroxides in charge reversal of protein-coated nanoparticles. Phys. Chem. Chem. Phys. 2019, 21, 11011–11018. [Google Scholar] [CrossRef] [Green Version]
- Google Images, Queried with the Phrase, Drawings of Nanoparticle Protein Coronas. Available online: https://www.google.com/search?q=Drawings+of+Nanoparticle+Protein+Corona&client=firefox-b-d&source=lnms&tbm=isch&sa=X&ved=2ahUKEwiZgq-ygrT0AhWQj4kEHSypDasQ_AUoAXoECAIQAw&biw=1366&bih=626&dpr=1: (accessed on 31 October 2021).
- Google Images, Queried with the Phrase, TEM Photomicrographs of Nanoparticle Protein Coronas. Available online: https://www.google.com/search?q=TEM+Photomicrographs+of+Nanoparticle+Protein+Coronas&client=firefox-b-d&source=lnms&tbm=isch&sa=X&ved=2ahUKEwjOv5aCg7T0AhUUkYkEHaWRAqIQ_AUoAXoECAIQAw&biw=1366&bih=626&dpr=1: (accessed on 31 October 2021).
- Shannahan, J.H.; Lai, X.; Ke, P.C.; Podila, R.; Brown, J.M.; Witzmann, F.A. Silver Nanoparticle Protein Corona Composition in Cell Culture Media. PLoS ONE 2013, 8, e74001. [Google Scholar] [CrossRef] [PubMed]
- Eigenheer, R.; Castellanos, E.R.; Nakamoto, M.Y.; Gerner, K.T.; Lampe, A.M.; Wheeler, K.E. Silver nanoparticle protein corona composition compared across engineered particle properties and environmentally relevant reaction conditions. Environ. Sci. Nano 2014, 1, 238–247. [Google Scholar] [CrossRef]
- Durán, N.; Silveira, C.P.; Durán, M.; Martinez, D.S.T. Silver nanoparticle protein corona and toxicity: A mini-review. J. Nanobiotechnol. 2015, 13, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbo, C.; Molinaro, R.; Parodi, A.; Furman, N.E.T.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.H.; Lee, B.-J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Lin, L.; Wei, A.; Sepúlveda, M.S. Protein Corona Analysis of Silver Nanoparticles exposed to Fish Plasma. Environ. Sci. Technol. Lett. 2017, 4, 174–179. [Google Scholar] [CrossRef]
- Gorshkov, V.; Bubis, J.A.; Solovyeva, E.M.; Gorshkov, M.V.; Kjeldsen, F. Protein corona formed on silver nanoparticles is highly selective and resistant to physicochemical changes of the solution. Environ. Sci. Nano 2019, 6, 1089–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad-Beigi, H.; Hayashi, Y.; Zeuthen, C.M.; Eskandari, H.; Scavenius, C.; Juul-Madsen, K.; Vorup-Jensen, T.; Enghild, J.J.; Sutherland, D.S. Mapping and identification of soft corona proteins at nanoparticles and their impact on cellular association. Nat. Commun. 2020, 11, 4535. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, R.; Vallet-Regí, M. Hard and Soft Protein Corona of Nanomaterials: Analysis and Relevance. Nanomaterials 2021, 11, 888. [Google Scholar] [CrossRef]
- Bai, X.; Wang, J.; Mu, Q.; Su, G. In Vivo Protein Corona Formation: Characterization, Effects on Engineered Particles’ Biobehaviors, and Applications. Front. Bioeng. Biotechnol. 2021, 9, 686708. [Google Scholar] [CrossRef] [PubMed]
- Kang, F.; Alvarez, P.J.; Zhu, D. Microbial extracellular polymeric substances reduce Ag+ to silver nanoparticles and antagonize bactericidal activity. Environ. Sci. Technol. 2014, 48, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, E.A.; Poverennaya, E.V.; Ilgisonis, E.V.; Pyatnitskiy, M.; Kopylov, A.; Zgoda, V.G.; Lisitsa, A.V.; Archakov, A.I. The Size of the Human Proteome: The Width and Depth. Int. J. Anal. Chem. 2016, 2016, 7436849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Google Images, Queried with the Phrase, TEM Photomicrographs of Bacteria in Blood. Available online: https://www.google.com/search?q=TEM+Photomicrographs+of+Bacteria+in+Blood&client=firefox-b-d&source=lnms&tbm=isch&sa=X&ved=2ahUKEwje1p3RjbT0AhV_k4kEHRCXChIQ_AUoAXoECAEQAw&biw=1366&bih=626&dpr=1: (accessed on 31 October 2021).
- Available online: https://bio.libretexts.org/Bookshelves/Microbiology/Book%3A_Microbiology_(Bruslind)/04%3A_Bacteria%3A_Cell_Walls (accessed on 31 October 2021).
- Silhavyl, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol. 2010, 2, a000414. [Google Scholar]
- Buynak, J.D. Cutting and Stitching: The Cross-Linking of Peptidoglycan in the Assembly of the Bacterial Cell Wall. ACS Chem. Biol. 2007, 2, 602–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.-Q.; Solmont Technology Wuxi Co., Ltd., Wuxi, China. Personal Communication, 2021.
- Scott, D.A. Metallography and Microstructure in Ancient and Historic Metals; Getty Publications: Singapore, 1992; p. 12. [Google Scholar]
- Li, A.; Szlufarska, I. Morphology and mechanical properties of nanocrystalline Cu/Ag alloy. J. Mater. Sci. 2017, 52, 4555–4567. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Ye, J.; Pan, Z.; Geng, J.; Besser, M.F.; Qu, D.-X.; Caro, A.; Marian, J.; Ott, R.T.; Wang, Y.M.; et al. Ideal maximum strengths and defect-induced softening in nanocrystalline-nanotwinned metals. Nat. Mater. 2019, 18, 1207–1214. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sunada, K.; Nagai, T.; Isobe, T.; Matsushita, S.; Ishiguro, H.; Nakajima, A. Preparation of hydrophobic La2Mo2O9 ceramics with antibacterial and antiviral properties. J. Hazard. Mater. 2019, 378, 120610. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Sunada, K.; Nagai, T.; Isobe, T.; Matsushita, S.; Ishiguro, H.; Nakajima, A. Effects of cerium and tungsten substitution on antiviral and antibacterial properties of lanthanum molybdate. Mater. Sci. Eng. C 2020, 117, 111323. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sunada, K.; Nagai, T.; Ishiguro, H.; Nakano, R.; Suzuki, Y.; Nakano, A.; Yano, H.; Isobe, T.; Matsushita, S.; et al. Preparation of cerium molybdates and their antiviral activity against bacteriophages Φ6 and SARS-CoV-2. Mater. Lett. 2021, 290, 129510. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, L.; Guo, C.; Yan, B.; Su, G. Regulating Protein Corona Formation and Dynamic Protein Exchange by Controlling Nanoparticle Hydrophobicity. Front. Bioeng. Biotechnol. 2020, 8, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J. Protein-Nanoparticle Interaction: Corona Formation and Conformational Changes in Proteins on Nanoparticles. Int. J. Nanomed. 2020, 15, 5783–5802. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of nanoparticles with proteins: Relation to bio-reactivity of the nanoparticle. J. Nanobiotech. 2013, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Sápi, A.; Rajkumar, T.; Kiss, J.; Kukovecz, Á.; Kónya, Z.; Somorjai, G.A. Metallic Nanoparticles in Heterogeneous Catalysis. Catal. Lett. 2021, 151, 2153–2175. [Google Scholar] [CrossRef]
- Adair, J.H.; Suvaci, E.; Sindel, J. Surface and Colloid Chemistry. In Encyclopedia of Materials Science and Technology, 2nd ed.; Jürgen Buschow, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Eds.; Elsevier: London, UK, 2001. [Google Scholar]
- Barbalinardo, M.; Bertacchini, J.; Bergamini, L.; Magarò, M.S.; Ortolani, L.; Sanson, A.; Palumbo, C.; Cavallini, M.; Gentili, D. Surface Properties modulate protein corona formation and determine cellular uptake and cytotoxicity of silver nanoparticles. Nanoscale 2021, 13, 14119–14129. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef] [PubMed]
- França, R.; Zhang, X.F.; Veres, T.; Yahia, L.’H.; Sacher, E. Core-shell nanoparticles as prodrugs: Possible cytotoxicological and biomedical impacts of batch-to-batch inconsistencies. J. Colloid Interface Sci. 2013, 389, 292–297. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacher, E.; Yelon, A. A Pragmatic Perspective of the Antibacterial Properties of Metal-Based Nanoparticles. Nanomaterials 2021, 11, 3214. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123214
Sacher E, Yelon A. A Pragmatic Perspective of the Antibacterial Properties of Metal-Based Nanoparticles. Nanomaterials. 2021; 11(12):3214. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123214
Chicago/Turabian StyleSacher, Edward, and Arthur Yelon. 2021. "A Pragmatic Perspective of the Antibacterial Properties of Metal-Based Nanoparticles" Nanomaterials 11, no. 12: 3214. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123214