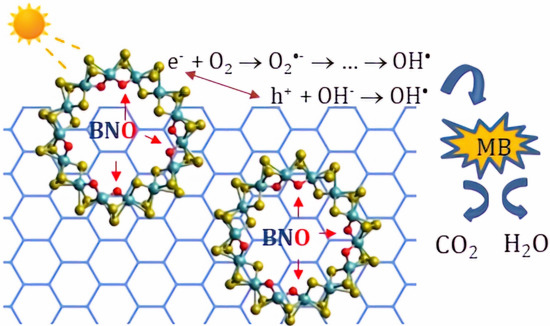
Amorphous MoSxOy/h-BNxOy Nanohybrids: Synthesis and Dye Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Fabrication of a-MoSxOy/h-BNxOy Nanohybrids
2.3. Material Characterization
2.4. Photocatalytic Measurements
2.5. DFT Analysis
3. Results
3.1. Characterization of BNO and a-MoSxOy/h-BNxOy Materials
3.2. Photocatalytic Activity
3.3. Theoretical Investigation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amoatey, P.; Baawain, M.S. Effects of Pollution on Freshwater Aquatic Organisms. Water Environ. Res. 2019, 91, 1272–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbe, H.; Nyankson, E.; Raza, N.; Dodoo-Arhin, D.; Chauhan, A.; Osei, G.; Kumar, V.; Kim, K.-H. Recent Advances in Photoinduced Catalysis for Water Splitting and Environmental Applications. J. Ind. Eng. Chem. 2019, 72, 31–49. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, Z.; Khan, A.U.; Mastoi, N.R.; Aslam, M.; Kim, J. Photocatalytic Systems as an Advanced Environmental Remediation: Recent Developments, Limitations and New Avenues for Applications. J. Environ. Chem. Eng. 2016, 4, 4143–4164. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-Based Photocatalysts and Photoelectrochemical Cells for Solar Fuel Generation: A Review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic Hydrogen Evolution: MoS2 Nanoparticles as Catalyst for Hydrogen Evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum Sulfides—Efficient and Viable Materials for Electro–and Photoelectrocatalytic Hydrogen Evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef]
- Tran, P.D.; Tran, T.V.; Orio, M.; Torelli, S.; Truong, Q.D.; Nayuki, K.; Sasaki, Y.; Chiam, S.Y.; Yi, R.; Honma, I.; et al. Coordination Polymer Structure and Revisited Hydrogen Evolution Catalytic Mechanism for Amorphous Molybdenum Sulfide. Nat. Mater. 2016, 15, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Pető, J.; Ollár, T.; Vancsó, P.; Popov, Z.I.; Magda, G.Z.; Dobrik, G.; Hwang, C.; Sorokin, P.B.; Tapasztó, L. Spontaneous Doping of the Basal Plane of MoS2 Single Layers through Oxygen Substitution under Ambient Conditions. Nat. Chem. 2018, 10, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Benck, J.D.; Chen, Z.; Kuritzky, L.Y.; Forman, A.J.; Jaramillo, T.F. Amorphous Molybdenum Sulfide Catalysts for Electrochemical Hydrogen Production: Insights into the Origin of Their Catalytic Activity. ACS Catal. 2012, 2, 1916–1923. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, M.; Reddy, G.B. Oxidized Core–Shell MoO2–MoS2 Nanostructured Thin Films for Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Chen, I.-W.P.; Lai, Y.-M.; Liao, W.-S. One-Pot Synthesis of Chlorophyll-Assisted Exfoliated MoS2/WS2 Heterostructures via Liquid-Phase Exfoliation Method for Photocatalytic Hydrogen Production. Nanomaterials 2021, 11, 2436. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Longo, A.; Dzade, N.Y.; Sharma, A.; Hendrix, M.M.R.M.; Bol, A.A.; de Leeuw, N.H.; Hensen, E.J.M.; Hofmann, J.P. The Origin of High Activity of Amorphous MoS2 in the Hydrogen Evolution Reaction. ChemSusChem 2019, 12, 4383–4389. [Google Scholar] [CrossRef] [Green Version]
- Le, D.; Rawal, T.B.; Rahman, T.S. Single-Layer MoS2 with Sulfur Vacancies: Structure and Catalytic Application. J. Phys. Chem. C 2014, 118, 5346–5351. [Google Scholar] [CrossRef]
- Singh, N.; Jabbour, G.; Schwingenschlögl, U. Optical and Photocatalytic Properties of Two-Dimensional MoS2. Eur. Phys. J. B 2012, 85, 392. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.-L.; Araujo, C.M.; Luo, W.; Ahuja, R. Single-Layer MoS2 as an Efficient Photocatalyst. Catal. Sci. Technol. 2013, 3, 2214–2220. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Shao, C.; Li, X.; Miao, F.; Wang, K.; Lu, N.; Liu, Y. 3D MoS2 Nanosheet/TiO2 Nanofiber Heterostructures with Enhanced Photocatalytic Activity under UV Irradiation. J. Alloy. Compd. 2016, 686, 137–144. [Google Scholar] [CrossRef]
- Pesci, F.M.; Sokolikova, M.S.; Grotta, C.; Sherrell, P.C.; Reale, F.; Sharda, K.; Ni, N.; Palczynski, P.; Mattevi, C. MoS2/WS2 Heterojunction for Photoelectrochemical Water Oxidation. ACS Catal. 2017, 7, 4990–4998. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, B.; Ghorbani-Asl, M.; Kretschmer, S.; Ghosh, A.; Guha, P.; Panda, S.K.; Jena, B.; Krasheninnikov, A.V.; Jena, B.K. MoS2 Quantum Dots as Efficient Catalyst Materials for the Oxygen Evolution Reaction. ACS Catal. 2018, 8, 1683–1689. [Google Scholar] [CrossRef]
- Lalithambika, K.C.; Shanmugapriya, K.; Sriram, S. Photocatalytic Activity of MoS2 Nanoparticles: An Experimental and DFT Analysis. Appl. Phys. A 2019, 125, 817. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Liu, H.; Wang, X.-X.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. One-Step Low Temperature Hydrothermal Synthesis of Flexible TiO2/PVDF@MoS2 Core-Shell Heterostructured Fibers for Visible-Light-Driven Photocatalysis and Self-Cleaning. Nanomaterials 2019, 9, 431. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wu, W.; Fang, Y.; Liu, H.; Chen, F.; Zhang, M.; Qin, Y. Functionalized MoS2 Nanoflowers with Excellent Near-Infrared Photothermal Activities for Scavenging of Antibiotic Resistant Bacteria. Nanomaterials 2021, 11, 2829. [Google Scholar] [CrossRef]
- Hasija, V.; Raizada, P.; Thakur, V.K.; Parwaz Khan, A.A.; Asiri, A.M.; Singh, P. An Overview of Strategies for Enhancement in Photocatalytic Oxidative Ability of MoS2 for Water Purification. J. Environ. Chem. Eng. 2020, 8, 104307. [Google Scholar] [CrossRef]
- Huang, W.-H.; Li, X.-M.; Yang, X.-F.; Zhang, H.-B.; Wang, F.; Zhang, J. Highly Efficient Electrocatalysts for Overall Water Splitting: Mesoporous CoS/MoS2 with Hetero-Interfaces. Chem. Commun. 2021, 57, 4847–4850. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hao, J.; Lau, S.P. Synthesis, Properties, and Applications of 2D Amorphous Inorganic Materials. J. Appl. Phys. 2020, 127, 220901. [Google Scholar] [CrossRef]
- Chiawchan, T.; Ramamoorthy, H.; Buapan, K.; Somphonsane, R. CVD Synthesis of Intermediate State-Free, Large-Area and Continuous MoS2 via Single-Step Vapor-Phase Sulfurization of MoO2 Precursor. Nanomaterials 2021, 11, 2642. [Google Scholar] [CrossRef]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous Molybdenum Sulfide Films as Catalysts for Electrochemical Hydrogen Production in Water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Morales-Guio, C.G.; Hu, X. Amorphous Molybdenum Sulfides as Hydrogen Evolution Catalysts. Acc. Chem. Res. 2014, 47, 2671–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinda, D.; Ahmed, M.E.; Mandal, S.; Mondal, B.; Saha, S.K. Amorphous Molybdenum Sulfide Quantum Dots: An Efficient Hydrogen Evolution Electrocatalyst in Neutral Medium. J. Mater. Chem. A 2016, 4, 15486–15493. [Google Scholar] [CrossRef]
- Mishra, N.S.; Saravanan, P. A Review on the Synergistic Features of Hexagonal Boron Nitride (White Graphene) as Adsorbent-Photo Active Nanomaterial. ChemistrySelect 2018, 3, 8023–8034. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, A.; He, W.; Xing, Y.; Xu, X. 2D New Nonmetal Photocatalyst of Sulfur-Doped h-BN Nanosheeets with High Photocatalytic Activity. Adv. Mater. Interfaces 2019, 6, 1900062. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z. 2D MoS2 Nanostructures for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1701158. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, H.; Tang, C.; Lei, S.; Shen, W.; Wang, C.; Wang, G.; Wang, Z.; Wang, L. Toxicity Evaluation of Boron Nitride Nanospheres and Water-Soluble Boron Nitride in Caenorhabditis Elegans. Int. J. Nanomed. 2017, 12, 5941–5957. [Google Scholar] [CrossRef] [Green Version]
- Gerrard, W. Solubility of Hydrogen Sulphide, Dimethyl Ether, Methyl Chloride and Sulphur Dioxide in Liquids. The Prediction of Solubility of All Gases. J. Appl. Chem. Biotechnol. 2007, 22, 623–650. [Google Scholar] [CrossRef]
- Hunger, J.; Buchner, R.; Kandil, M.E.; May, E.F.; Marsh, K.N.; Hefter, G. Relative Permittivity of Dimethylsulfoxide and N,N-Dimethylformamide at Temperatures from (278 to 328) K and Pressures from (0.1 to 5) MPa. J. Chem. Eng. Data 2010, 55, 2055–2065. [Google Scholar] [CrossRef]
- Endler, I.; Leonhardt, A.; König, U.; van den Berg, H.; Pitschke, W.; Sottke, V. Chemical Vapour Deposition of MoS2 Coatings Using the Precursors MoCl5 and H2S. Surf. Coat. Technol. 1999, 120–121, 482–488. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Shimojo, F.; Hoshino, K.; Zempo, Y. Ab Initio Molecular-Dynamics Simulation Method for Complex Liquids. Comput. Phys. Commun. 2001, 142, 364–367. [Google Scholar] [CrossRef]
- Ren, J.; Stagi, L.; Innocenzi, P. Hydroxylated Boron Nitride Materials: From Structures to Functional Applications. J. Mater. Sci. 2021, 56, 4053–4079. [Google Scholar] [CrossRef]
- Matveev, A.T.; Permyakova, E.S.; Kovalskii, A.M.; Leibo, D.; Shchetinin, I.V.; Maslakov, K.I.; Golberg, D.V.; Shtansky, D.V.; Konopatsky, A.S. New Insights into Synthesis of Nanocrystalline Hexagonal BN. Ceram. Int. 2020, 46, 19866–19872. [Google Scholar] [CrossRef]
- Deng, Y.; Ting, L.R.L.; Neo, P.H.L.; Zhang, Y.-J.; Peterson, A.A.; Yeo, B.S. Operando Raman Spectroscopy of Amorphous Molybdenum Sulfide (MoSx) during the Electrochemical Hydrogen Evolution Reaction: Identification of Sulfur Atoms as Catalytically Active Sites for H+ Reduction. ACS Catal. 2016, 6, 7790–7798. [Google Scholar] [CrossRef]
- Müller, A.; Jaegermann, W.; Enemark, J.H. Disulfur Complexes. Coord. Chem. Rev. 1982, 46, 245–280. [Google Scholar] [CrossRef]
- Fedin, V.; Kolesov, B.; Mironov, Y.; Fedorov, V.Y. Synthesis and Vibrational (IR and Raman) Spectroscopic Study of Triangular Thio-Complexes [Mo3S13]2−Containing 92Mo, 100Mo and 34S Isotopes. Polyhedron 1989, 8, 2419–2423. [Google Scholar] [CrossRef]
- Fominski, V.; Demin, M.; Nevolin, V.; Fominski, D.; Romanov, R.; Gritskevich, M.; Smirnov, N. Reactive Pulsed Laser Deposition of Clustered-Type MoSx (x ~ 2, 3, and 4) Films and Their Solid Lubricant Properties at Low Temperature. Nanomaterials 2020, 10, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of Disorder on Raman Scattering of Single-Layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef] [Green Version]
- Griffith, W.P. Raman Studies on Rock-Forming Minerals. Part II. Minerals Containing MO3, MO4, and MO6 Groups. J. Chem. Soc. Inorg. Phys. Theor. 1970, 286–291. [Google Scholar] [CrossRef]
- Simon, G.; Hehlen, B.; Vacher, R.; Courtens, E. Hyper-Raman Scattering Analysis of the Vibrations in Vitreous Boron Oxide. Phys. Rev. B 2007, 76, 054210. [Google Scholar] [CrossRef] [Green Version]
- Gouin, X.; Grange, P.; Bois, L.; L’Haridon, P.; Laurent, Y. Characterization of the Nitridation Process of Boric Acid. J. Alloy. Compd. 1995, 224, 22–28. [Google Scholar] [CrossRef]
- Weber, T.; Muijsers, J.C.; Niemantsverdriet, J.W. Structure of Amorphous MoS3. J. Phys. Chem. 1995, 99, 9194–9200. [Google Scholar] [CrossRef] [Green Version]
- Xi, F.; Bogdanoff, P.; Harbauer, K.; Plate, P.; Höhn, C.; Rappich, J.; Wang, B.; Han, X.; van de Krol, R.; Fiechter, S. Structural Transformation Identification of Sputtered Amorphous MoSx as an Efficient Hydrogen-Evolving Catalyst during Electrochemical Activation. ACS Catal. 2019, 9, 2368–2380. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Jaramillo, T.F.; Besenbacher, F. Building an Appropriate Active-Site Motif into a Hydrogen-Evolution Catalyst with Thiomolybdate [Mo3S13]2− Clusters. Nat. Chem. 2014, 6, 248–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, R.; Wang, G.; Jiang, C.; Tang, H.; Xu, Q. In Situ Hydrothermal Synthesis of G-C3N4/TiO2 Heterojunction Photocatalysts with High Specific Surface Area for Rhodamine B Degradation. Appl. Surf. Sci. 2017, 411, 400–410. [Google Scholar] [CrossRef]
- Lin, J.; Luo, Z.; Liu, J.; Li, P. Photocatalytic Degradation of Methylene Blue in Aqueous Solution by Using ZnO-SnO2 Nanocomposites. Mater. Sci. Semicond. Process. 2018, 87, 24–31. [Google Scholar] [CrossRef]
- Solehudin, M.; Sirimahachai, U.; Ali, G.A.M.; Chong, K.F.; Wongnawa, S. One-Pot Synthesis of Isotype Heterojunction g-C3N4-MU Photocatalyst for Effective Tetracycline Hydrochloride Antibiotic and Reactive Orange 16 Dye Removal. Adv. Powder Technol. 2020, 31, 1891–1902. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, H.; Wang, W.; Zhang, Y.; Li, Z.; Wu, X.; Yu, X.; Zhou, W. Fabrication of 3D Mesoporous Black TiO2/MoS2/TiO2 Nanosheets for Visible-Light-Driven Photocatalysis. ChemSusChem 2016, 9, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Laouini, S.E.; Bouafia, A.; Soldatov, A.V.; Algarni, H.; Tedjani, M.L.; Ali, G.A.M.; Barhoum, A. Green Synthesized of Ag/Ag2O Nanoparticles Using Aqueous Leaves Extracts of Phoenix Dactylifera L. and Their Azo Dye Photodegradation. Membranes 2021, 11, 468. [Google Scholar] [CrossRef]
- Lakshmi, S.; Renganathan, R.; Fujita, S. Study on TiO2-Mediated Photocatalytic Degradation of Methylene Blue. J. Photochem. Photobiol. Chem. 1995, 88, 163–167. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A Fast and Robust Algorithm for Bader Decomposition of Charge Density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Sahu, A.; Steinmann, S.N.; Raybaud, P. Size-Dependent Structural, Energetic, and Spectroscopic Properties of MoS3 Polymorphs. Cryst. Growth Des. 2020, 20, 7750–7760. [Google Scholar] [CrossRef]
- Cheriyan, S.; Balamurgan, D.; Sriram, S. Doping Effect on Monolayer MoS2 for Visible Light Dye Degradation—A DFT Study. Superlattices Microstruct. 2018, 116, 238–243. [Google Scholar] [CrossRef]







| Material | Binding Energy (eV) | |||||||
|---|---|---|---|---|---|---|---|---|
| B 1s | N 1s | O 1s | ||||||
| h-BNxOy | 192.8 | 191.0 | 190.2 | 399.0 | 398.0 | 534.8 | 536.6 | |
| a-MoSxOy/h-BNO | 191.7 | 190.6 | 398.8 | 398.2 | 532.9 | 531.0 | ||
| Binding Energy (eV) | |||||||
|---|---|---|---|---|---|---|---|
| Mo(6+) | Mo(5+) | Mo(4+) | S 2s | ||||
| 3d3/2 | 3d5/2 | 3d3/2 | 3d5/2 | 3d3/2 | 3d5/2 | ||
| 237.9 | 234.8 | 236.3 | 233.1 | 232.9 | 229.8 | 227.0 | 226.1 |
| Binding Energy (eV) | |||||
|---|---|---|---|---|---|
| S 2p | S (I) | S (II) | |||
| 2p1/2 | 2p3/2 | 2p1/2 | 2p3/2 | ||
| 170.2 | 168.8 | 164.9 | 162.3 | 165.3 | 163.8 |
| Catalytic System, (g L−1) | Dye, (g L−1) | Lamp | Specific Catalyst Mass Activity (mmol g−1 h−1) | Reference |
|---|---|---|---|---|
| MoS2/TiO2 *) (0.045) | RhB (0.01) | high pressure Hg lamp, 50 W | 0.766 | [18] |
| MoS2/TiO2 *) (0.045) | MO (0.01) | λ = 313 nm | 1.33 | [18] |
| Nanosheets S-BN (0.5) | RhB (0.02) | Xenon lamp, 500 W, λ > 400 nm | 0.03 | [32] |
| g-C3N4/TiO2 (0.04) | RhB (0.11) | Xenon arc, 350 W, λ > 420 nm | 0.188 | [54] |
| Nano (ZnO-SnO2) (0.2) | MB (0.02) | Hg lamp, 250 W | 0.005 | [55] |
| g-C3N4/MU (1.0) | RO16 (0.01) | Xenon lamp, 55 W, λ > 420 nm | 0.009 | [56] |
| MBTiO2/MoS2/TiO2 | MO (0.01) | Visible light | 0.014 | [57] |
| Ag/Ag2O nanoparticles (0.004) with NaBH4 | MB (~0.115) | - | ~85 | [58] |
| a-MoSxOy/h-BNxOy **) (0.006) | MB (0.02 g·L−1) | Hg lamp, 145 W | 5.51 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveev, A.T.; Konopatsky, A.S.; Leybo, D.V.; Volkov, I.N.; Kovalskii, A.M.; Varlamova, L.A.; Sorokin, P.B.; Fang, X.; Kulinich, S.A.; Shtansky, D.V. Amorphous MoSxOy/h-BNxOy Nanohybrids: Synthesis and Dye Photodegradation. Nanomaterials 2021, 11, 3232. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123232
Matveev AT, Konopatsky AS, Leybo DV, Volkov IN, Kovalskii AM, Varlamova LA, Sorokin PB, Fang X, Kulinich SA, Shtansky DV. Amorphous MoSxOy/h-BNxOy Nanohybrids: Synthesis and Dye Photodegradation. Nanomaterials. 2021; 11(12):3232. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123232
Chicago/Turabian StyleMatveev, Andrei T., Anton S. Konopatsky, Denis V. Leybo, Ilia N. Volkov, Andrey M. Kovalskii, Liubov A. Varlamova, Pavel B. Sorokin, Xiaosheng Fang, Sergei A. Kulinich, and Dmitry V. Shtansky. 2021. "Amorphous MoSxOy/h-BNxOy Nanohybrids: Synthesis and Dye Photodegradation" Nanomaterials 11, no. 12: 3232. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123232