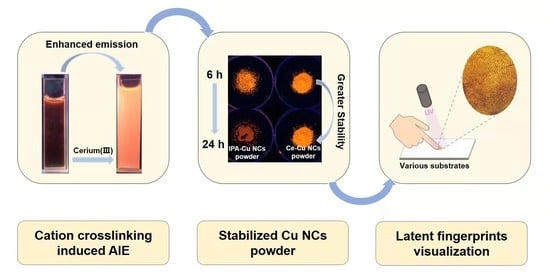
Cation Crosslinking-Induced Stable Copper Nanoclusters Powder as Latent Fingerprints Marker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Copper Nanoclusters (Cu NCs)
2.3. Synthesis and Stability Investigation of Cu NCs Powder by Solvent Precipitation Method and Cation Crosslinking Method
2.4. Latent Fingerprints Visualization Based on Ce-Cu NCs Powder
2.5. Characterizations
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Chen, B.; Rogach, A.L. Synthesis, optical properties and applications of light-emitting copper nanoclusters. Nanoscale Horiz. 2017, 2, 135–146. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y. Developing fluorescent copper nanoclusters: Synthesis, properties, and applications. Colloids Surf. B Biointerfaces 2020, 195, 111244. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, W.; Chen, W. Copper nanoclusters: Synthesis, characterization and properties. Chin. Sci. Bull. 2012, 57, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, B.; Susha, A.S.; Wang, W.; Reckmeier, C.J.; Chen, R.; Zhong, H.; Rogach, A.L. All-Copper Nanocluster Based Down-Conversion White Light-Emitting Devices. Adv. Sci. 2016, 3, 1600182. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, D.; Zhang, H. Self-Assembly Driven Aggregation-Induced Emission of Copper Nanoclusters: A Novel Technology for Lighting. ACS Appl. Mater. Interfaces 2018, 10, 12071–12080. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shao, C.; Li, L.; Liu, X.; Liu, M. In situ fabrication of a luminescent copper nanocluster/eggshell membrane composite and its application in visual detection of Ag+ ions, light-emitting diodes and surface patterning. Photochem. Photobiol. Sci. 2019, 18, 2942–2951. [Google Scholar] [CrossRef]
- Cai, Z.; Zhu, R.; Pang, S.; Tian, F.; Zhang, C. One-step Green Synthetic Approach for the Preparation of Orange Light Emitting Copper Nanoclusters for Sensitive Detection of Mercury(II) Ions. ChemistrySelect 2020, 5, 165–170. [Google Scholar] [CrossRef]
- Aparna, R.S.; Devi, J.A.; Nebu, J.; Syamchand, S.S.; George, S. Rapid response of dopamine towards insitu synthesised copper nanocluster in presence of H2O2. J. Photochem. Photobiol. A Chem. 2019, 379, 63–71. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Yang, Y.; Wang, C.; Zhou, Y.; Zhang, C.; Zhao, Y.; Yang, M.; Lin, Q. Ratiometric fluorescence detection of trace water in organic solvents based on aggregation-induced emission enhanced Cu nanoclusters. Analyst 2018, 143, 3068–3074. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Astruc, D. Atomically precise copper nanoclusters and their applications. Coord. Chem. Rev. 2018, 359, 112–126. [Google Scholar] [CrossRef]
- Gao, X.; Zhuang, X.; Tian, C.; Liu, H.; Lai, W.F.; Wang, Z.; Yang, X.; Chen, L.; Rogach, A.L. A copper nanocluster incorporated nanogel: Confinement-assisted emission enhancement for zinc ion detection in living cells. Sens. Actuators B Chem. 2020, 307, 127626. [Google Scholar] [CrossRef]
- Liu, Z.; Jing, X.; Zhang, S.; Tian, Y. A Copper Nanocluster-Based Fluorescent Probe for Real-Time Imaging and Ratiometric Biosensing of Calcium Ions in Neurons. Anal. Chem. 2019, 91, 2488–2497. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, Y.; Song, Q. Interfacial synthesis of polyethyleneimine-protected copper nanoclusters: Size-dependent tunable photoluminescence, pH sensor and bioimaging. Colloids Surf. B Biointerfaces 2016, 140, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Ao, H.; Feng, H.; Pan, S.; Bao, Z.; Li, Z.; Chen, J.; Qian, Z. Synthesis and Functionalization of Stable and Bright Copper Nanoclusters by In Situ Generation of Silica Shells for Bioimaging and Biosensing. ACS Appl. Nano Mater. 2018, 1, 5673–5681. [Google Scholar] [CrossRef]
- Qu, F.; Wang, B.; Li, K.; You, J.; Han, W. Copper nanoclusters@Al3+ complexes with strong and stable aggregation-induced emission for application in enzymatic determination of urea. Microchim. Acta 2020, 187, 457. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, L.; Wang, Y.; Hao, J. Stimuli-Responsive Fluorescent Nanoswitches: Solvent-Induced Emission Enhancement of Copper Nanoclusters. Chem.–Eur. J. 2020, 26, 3545–3554. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, H.; Liu, W.; Zhang, S.; Tang, C.; Chen, J.; Qian, Z. Cation-driven luminescent self-assembled dots of copper nanoclusters with aggregation-induced emission for β-galactosidase activity monitoring. J. Mater. Chem. B 2017, 5, 5120–5127. [Google Scholar] [CrossRef]
- You, J.G.; Lu, C.Y.; Kumar, A.S.K.; Tseng, W.L. Cerium(iii)-directed assembly of glutathione-capped gold nanoclusters for sensing and imaging of alkaline phosphatase-mediated hydrolysis of adenosine triphosphate. Nanoscale 2018, 10, 17691–17698. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Peng, T.; Li, Y.; Yu, M.; Hu, X.; He, G. Ultra-stable L-proline protected copper nanoclusters and their solvent effect. Methods Appl. Fluoresc. 2018, 6, 035015. [Google Scholar] [CrossRef]
- Zhao, M.; Qian, Z.; Zhong, M.; Chen, Z.; Ao, H.; Feng, H. Fabrication of Stable and Luminescent Copper Nanocluster-Based AIE Particles and Their Application in β-Galactosidase Activity Assay. ACS Appl. Mater. Interfaces 2017, 9, 32887. [Google Scholar] [CrossRef]
- Jia, X.; Yang, X.; Li, J.; Li, D.; Wang, E. Stable Cu nanoclusters: From an aggregation-induced emission mechanism to biosensing and catalytic applications. Chem. Commun. 2014, 50, 237–239. [Google Scholar] [CrossRef]
- Prakash, K.T.; Singh, N.; Venkatesh, V. Synthesis of novel luminescent copper nanoclusters with substituent driven self-assembly and aggregation induced emission (AIE). Chem. Commun. 2019, 55, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, R.; Xiong, Y.; Cepe, K.; Schneider, J.; Zboril, R.; Lee, C.S.; Rogach, A.L. Incorporating Copper Nanoclusters into Metal-Organic Frameworks: Confinement-Assisted Emission Enhancement and Application for Trinitrotoluene Detection. Part. Part. Syst. Charact. 2017, 34, 1700029. [Google Scholar] [CrossRef]
- Han, B.; Hu, X.; Yu, M.; Peng, T.; Li, Y.; He, G. One-pot synthesis of enhanced fluorescent copper nanoclusters encapsulated in metal–organic frameworks. RSC Adv. 2018, 8, 22748–22754. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Song, N.; Jia, Q. Investigation of the surface confinement effect of copper nanoclusters: Construction of an ultrasensitive fluorescence turn-on bio-enzyme sensing platform. Nanoscale 2019, 11, 21927–21933. [Google Scholar] [CrossRef]
- Ghubish, Z.; Saif, M.; Hafez, H.; Mahmoud, H.; Kamal, R.; El-Kemary, M. Novel red photoluminescence sensor based on Europium ion doped calcium hydroxy stannate CaSn(OH)6:Eu+3 for latent fingerprint detection. J. Mol. Struct. 2020, 1207, 127840. [Google Scholar] [CrossRef]
- Saif, M.; Alsayed, N.; Mbarek, A.; El-Kemary, M.; Abdel-Mottaleb, M.S.A. Preparation and characterization of new photoluminescent nano-powder based on Eu3+:La2Ti2O7 and dispersed into silica matrix for latent fingerprint detection. J. Mol. Struct. 2016, 1125, 763–771. [Google Scholar] [CrossRef]
- Wen, Z.; Song, S.; Wang, C.; Qu, F.; Thomas, T.; Hu, T.; Wang, P.; Yang, M. Large-scale synthesis of dual-emitting-based visualization sensing paper for humidity and ethanol detection. Sens. Actuators B Chem. 2019, 282, 9–15. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, Y.; Kershaw, S.V.; Chen, B.; Yang, X.; Goswami, N.; Lai, W.F.; Xie, J.; Rogach, A.L. In Situ Fabrication of Flexible, Thermally Stable, Large-Area, Strongly Luminescent Copper Nanocluster/Polymer Composite Films. Chem. Mater. 2017, 29, 10206–10211. [Google Scholar] [CrossRef]
- Wang, Z.; Susha, A.S.; Chen, B.; Reckmeier, C.; Tomanec, O.; Zboril, R.; Zhong, H.; Rogach, A.L. Poly(vinylpyrrolidone) supported copper nanoclusters: Glutathione enhanced blue photoluminescence for application in phosphor converted light emitting devices. Nanoscale 2016, 8, 7197–7202. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, G.; Cheng, L.; Wang, C.; Mei, X. Engineering the Self-Assembly Induced Emission of Copper Nanoclusters as 3D Nanomaterials with Mesoporous Sphere Structures by the Crosslinking of Ce3+. ACS Omega 2018, 3, 14755–14765. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Li, J.; Wang, E. Cu Nanoclusters with Aggregation Induced Emission Enhancement. Small 2013, 9, 3873–3879. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Ma, Y.; Wu, H.; Wang, X. Fluorescent and visual assay of H2O2 and glucose based on a highly sensitive copper nanoclusters-Ce(III) fluoroprobe. Anal. Bioanal. Chem. 2021, 413, 2135–2146. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, X.; Peng, Z.; Zhang, Y.; Liu, P.; Cai, Z.; Tong, B.; Shi, J.; Dong, Y. A “Turn-On” fluorescent chemosensor with the aggregation-induced emission characteristic for high-sensitive detection of Ce ion. Sens. Actuators B Chem. 2018, 267, 351–356. [Google Scholar] [CrossRef]
- Halawa, M.I.; Li, B.S.; Xu, G. Novel Synthesis of Thiolated Gold Nanoclusters Induced by Lanthanides for Ultrasensitive and Luminescent Detection of the Potential Anthrax Spores’ Biomarker. ACS Appl. Mater. Interfaces 2020, 12, 32888–32897. [Google Scholar] [CrossRef]
- Zhang, Q.; Mei, H.; Zhou, W.; Wang, X. Cerium ion(III)-triggered aggregation-induced emission of copper nanoclusters for trace-level p-nitrophenol detection in water. Microchem. J. 2021, 162, 105842. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhang, X.; Li, B.; Guo, R.; Cheng, Q.; Cheng, X. Synthesis of magnetic CuO/MnFe2O4 nanocompisite and its high activity for degradation of levofloxacin by activation of persulfate. Chem. Eng. J. 2019, 360, 848–860. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Peng, J.; Zhang, H.; Zhang, Y.; Lai, B. Efficient degradation of sulfamethoxazole by the CuO@Al2O3 (EPC) coupled PMS system: Optimization, degradation pathways and toxicity evaluation. Chem. Eng. J. 2019, 359, 1097–1110. [Google Scholar] [CrossRef]
- Ghodselahi, T.; Vesaghi, M.A.; Shafiekhani, A.; Baghizadeh, A.; Lameii, M. XPS study of the Cu@Cu2O core-shell nanoparticles. Appl. Surf. Sci. 2008, 255 Pt 2, 2730–2734. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, C.; Qi, K.; Cui, X. Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci. Rep. 2017, 7, 39695. [Google Scholar] [CrossRef] [PubMed]
- Orooji, Y.; Irani-Nezhad, M.H.; Hassandoost, R.; Khataee, A.; Pouran, S.R.; Joo, S.W. Cerium doped magnetite nanoparticles for highly sensitive detection of metronidazole via chemiluminescence assay. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118272. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Guo, Y.; Lin, M.; Yuan, M.; Liu, Z.; Huang, C. Optically active red-emitting Cu nanoclusters originating from complexation and redox reaction between copper(ii) and d/l-penicillamine. Nanoscale 2016, 8, 9764–9770. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Pradhan, N.; Panda, P.K.; Mishra, B.K. Biogenic unmodified gold nanoparticles for selective and quantitative detection of cerium using UV–vis spectroscopy and photon correlation spectroscopy (DLS). Biosens. Bioelectron. 2015, 68, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Wang, Z.; Zhang, Z.; Pu, F.; Ren, J.; Qu, X. Nucleic-acid-programmed Ag-nanoclusters as a generic platform for visualization of latent fingerprints and exogenous substances. Chem. Commun. 2016, 52, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, J.S.; Zhang, P.; Niu, X.Q.; Zhao, W.; Zhu, Z.Y.; Ding, H.; Xiong, H.M. Red-Emissive Carbon Dots for Fingerprints Detection by Spray Method: Coffee Ring Effect and Unquenched Fluorescence in Drying Process. ACS Appl. Mater. Interfaces 2017, 9, 18429–18433. [Google Scholar] [CrossRef]
- Zhai, Y.; Shen, F.; Zhang, X.; Jing, P.; Li, D.; Yang, X.; Zhou, D.; Xu, X.; Qu, S. Synthesis of green emissive carbon dots@montmorillonite composites and their application for fabrication of light-emitting diodes and latent fingerprints markers. J. Colloid Interface Sci. 2019, 554, 344–352. [Google Scholar] [CrossRef]
- Abdelwahab, W.M.; Phillips, E.; Patonay, G. Preparation of fluorescently labeled silica nanoparticles using an amino acid-catalyzed seeds regrowth technique: Application to latent fingerprints detection and hemocompatibility studies. J. Colloid Interface Sci. 2018, 512, 801–811. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Su, B. Aggregation induced emission for the recognition of latent fingerprints. Chem. Commun. 2012, 48, 4109–4111. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Yu, A.; Wu, J.; Mao, C. Rare Earth Fluorescent Nanomaterials for Enhanced Development of Latent Fingerprints. ACS Appl. Mater. Interfaces 2015, 7, 28110–28115. [Google Scholar] [CrossRef] [Green Version]
- Algarra, M.; Bartolić, D.; Radotić, K.; Mutavdžić, D.; Pino-González, M.S.; Rodríguez-Castellón, E.; Lázaro-Martínez, J.M.; Guerrero-González, J.J.; da Silva, J.C.E.; Jiménez-Jiménez, J. P-doped carbon nano-powders for fingerprint imaging. Talanta 2019, 194, 150–157. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Wen, Z.; Mei, S.; Wei, J.; Chen, Y.; Hu, Z.; Cui, Z.; Zhang, W.; Xie, F.; Guo, R. Cation Crosslinking-Induced Stable Copper Nanoclusters Powder as Latent Fingerprints Marker. Nanomaterials 2021, 11, 3371. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123371
Qiu Y, Wen Z, Mei S, Wei J, Chen Y, Hu Z, Cui Z, Zhang W, Xie F, Guo R. Cation Crosslinking-Induced Stable Copper Nanoclusters Powder as Latent Fingerprints Marker. Nanomaterials. 2021; 11(12):3371. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123371
Chicago/Turabian StyleQiu, Yi, Zhuoqi Wen, Shiliang Mei, Jinxin Wei, Yuanyuan Chen, Zhe Hu, Zhongjie Cui, Wanlu Zhang, Fengxian Xie, and Ruiqian Guo. 2021. "Cation Crosslinking-Induced Stable Copper Nanoclusters Powder as Latent Fingerprints Marker" Nanomaterials 11, no. 12: 3371. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123371