The Effects of the Binder and Buffering Matrix on InSb-Based Anodes for High-Performance Rechargeable Li-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of InSb and InSb–C
2.2. Material Characterization
2.3. Electrochemical Measurements
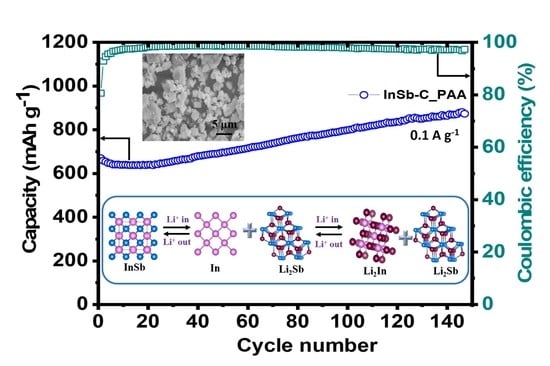
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of lithium-ion batteries in grid-scale energy storage systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Kong, S.; Li, R.; Hong, Z.; Huang, F.Q. Pyrochlore phase Ce2Sn2O7via an atom-confining strategy for reversible lithium storage. J. Mater. Chem. A 2020, 8, 5744–5749. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Meng, J.; Guo, H.; Niu, C.; Zhao, Y.; Xu, L.; Li, Q.; Mai, L. Advances in structure and property optimizations of battery electrode materials. Joule 2017, 1, 522–547. [Google Scholar] [CrossRef] [Green Version]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Jiao, X.; Liu, Y.; Li, B.; Zhang, W.; He, C.; Zhang, C.; Yu, Z.; Gao, T.; Song, J. Amorphous phosphorus-carbon nanotube hybrid anode with ultralong cycle life and high-rate capability for lithium-ion batteries. Carbon 2019, 148, 518–524. [Google Scholar] [CrossRef]
- Mun, Y.S.; Pham, T.N.; Bui, V.K.H.; Tanaji, S.T.; Lee, H.U.; Lee, G.-W.; Choi, J.S.; Kim, I.T.; Lee, Y.-C. Tin oxide evolution by heat-treatment with tin-aminoclay (SnAC) under argon condition for lithium-ion battery (LIB) anode applications. J. Power Sources 2019, 437, 226946. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Park, D.; Kim, I.T. FexSnyOz composites as anode materials for lithium-ion storage. J. Nanosci. Nanotechnol. 2019, 19, 6636–6640. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Kim, J.H.; Kim, I.T. Electrochemical performance of Sn/SnO/Ni3Sn composite anodes for lithium-ion batteries. J. Nanosci. Nanotechnol. 2019, 19, 1001–1005. [Google Scholar] [CrossRef]
- Pham, T.N.; Tanaji, S.T.; Choi, J.S.; Lee, H.U.; Kim, I.T.; Lee, Y.C. Preparation of Sn-aminoclay (SnAC)-templated Fe3O4 nanoparticles as an anode material for lithium-ion batteries. RSC Adv. 2019, 9, 10536–10545. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Kim, I. Ag nanoparticle-decorated MoS2 nanosheets for enhancing electrochemical performance in lithium storage. Nanomaterials 2021, 11, 626. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Kim, I.T. Self-assembled few-layered MoS2 on SnO2 anode for enhancing lithium-ion storage. Nanomaterials 2020, 10, 2558. [Google Scholar] [CrossRef]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S.-K. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. W2C/WS2 alloy nanoflowers as anode materials for lithium-ion storage. Nanomaterials 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Vo, T.N.; Kim, I.T. GeTe-TiC-C composite anodes for li-ion storage. Materials 2020, 13, 4222. [Google Scholar] [CrossRef]
- Vo, T.N.; Kim, D.S.; Mun, Y.S.; Lee, H.J.; Ahn, S.-K.; Kim, I.T. Fast charging sodium-ion batteries based on Te-P-C composites and insights to low-frequency limits of four common equivalent impedance circuits. Chem. Eng. J. 2020, 398, 125703. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef]
- Lai, S.Y.; Knudsen, K.D.; Sejersted, B.T.; Ulvestad, A.; Mæhlen, J.P.; Koposov, A.Y. Silicon nanoparticle ensembles for lithium-ion batteries elucidated by small-angle neutron scattering. ACS Appl. Energy Mater. 2019, 2, 3220–3227. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, L.; Li, Y.; Li, Y.; Lee, H.R.; Pei, A.; He, X.; Cui, Y. Design of red phosphorus nanostructured electrode for fast-charging lithium-ion batteries with high energy density. Joule 2019, 3, 1080–1093. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Feng, J.; Bian, X.; Qian, Y.; Liu, J.; Xu, H. Nanoporous germanium as high-capacity lithium-ion battery anode. Nano Energy 2015, 13, 651–657. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xia, Y. A high performance lithium-ion sulfur battery based on a Li2S cathode using a dual-phase electrolyte. Energy Environ. Sci. 2015, 8, 1551–1558. [Google Scholar] [CrossRef]
- Nam, K.-H.; Park, C.-M. Layered Sb2Te3 and its nanocomposite: A new and outstanding electrode material for superior rechargeable Li-ion batteries. J. Mater. Chem. A 2016, 4, 8562–8565. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Yang, Y.; Zhu, Y.; Fang, L.; Song, W.; Pan, C.; Yang, X.; Ji, X. Sodium/lithium storage behavior of antimony hollow nanospheres for rechargeable batteries. ACS Appl. Mater. Interfaces 2014, 6, 16189–16196. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, F.; Gaumet, J.-J.; Magri, P.; Diliberto, S.; Zhou, L.; Mai, L. Bottom-up confined synthesis of nanorod-in-nanotube structured Sb–N–C for durable lithium and sodium storage. Adv. Energy Mater. 2018, 8, 1703237. [Google Scholar] [CrossRef]
- Nam, K.-H.; Park, C.-M. 2D layered Sb2Se3-based amorphous composite for high-performance Li- and Na-ion battery anodes. J. Power Sources 2019, 433, 126639. [Google Scholar] [CrossRef]
- Darwiche, A.; Marino, C.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Better cycling performances of bulk Sb in Na-Ion batteries compared to Li-Ion Systems: An unexpected electrochemical mechanism. J. Am. Chem. Soc. 2012, 134, 20805–20811. [Google Scholar] [CrossRef]
- Yi, Z.; Han, Q.; Geng, D.; Wu, Y.; Cheng, Y.; Wang, L. One-pot chemical route for morphology-controllable fabrication of Sn-Sb micro/nano-structures: Advanced anode materials for lithium and sodium storage. J. Power Sources 2017, 342, 861–871. [Google Scholar] [CrossRef]
- Hou, H.; Jing, M.; Yang, Y.; Zhang, Y.; Song, W.; Yang, X.; Chen, J.; Chen, Q.; Ji, X. Antimony nanoparticles anchored on interconnected carbon nanofibers networks as advanced anode material for sodium-ion batteries. J. Power Sources 2015, 284, 227–235. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.-Y.; Lou, X.W.; Paik, U. Sb–C coaxial nanotubes as a superior long-life and high-rate anode for sodium ion batteries. Energy Environ. Sci. 2016, 9, 2314–2318. [Google Scholar] [CrossRef]
- Zhao, X.; Vail, S.A.; Lu, Y.; Song, J.; Pan, W.; Evans, D.R.; Lee, J.-J. Antimony/graphitic carbon composite anode for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 13871–13878. [Google Scholar] [CrossRef]
- Yi, Z.; Han, Q.; Zan, P.; Wu, Y.; Cheng, Y.; Wang, L. Sb nanoparticles encapsulated into porous carbon matrixes for high-performance lithium-ion battery anodes. J. Power Sources 2016, 331, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Zhou, J.; Zhang, G.; Guo, C.; Li, M.; Zhu, Y.; Qian, Y. Sb nanoparticles uniformly dispersed in 1-D N-doped porous carbon as anodes for Li-ion and Na-ion batteries. J. Mater. Chem. A 2017, 5, 12144–12148. [Google Scholar] [CrossRef]
- Wan, F.; Guo, J.-Z.; Zhang, X.-H.; Zhang, J.-P.; Sun, H.-Z.; Yan, Q.; Han, D.-X.; Niu, L.; Wu, X.-L. In situ binding Sb nanospheres on graphene via oxygen bonds as superior anode for ultrafast sodium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 7790–7799. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, W.; Yang, Z.; Gu, L.; Yu, Y. Nanoconfined antimony in sulfur and nitrogen co-doped three-dimensionally (3D) interconnected macroporous carbon for high-performance sodium-ion batteries. Nano Energy 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Yim, T.; Choi, S.J.; Jo, Y.N.; Kim, T.-H.; Kim, K.J.; Jeong, G.; Kim, Y.-J. Effect of binder properties on electrochemical performance for silicon-graphite anode: Method and application of binder screening. Electrochim. Acta 2014, 136, 112–120. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, T.-W.; Coskun, A.; Choi, J.W. Highly elastic binders integrating polyrotaxanes for silicon microparticle anodes in lithium ion batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Xiao, X.; Vukmirovic, N.; Xun, S.; Das, P.K.; Song, X.; Olalde-Velasco, P.; Wang, D.; Weber, A.Z.; Wang, L.-W.; et al. Toward an ideal polymer binder design for high-capacity battery anodes. J. Am. Chem. Soc. 2013, 135, 12048–12056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, B.; Kim, H.; Cho, Y.; Lee, K.T.; Choi, N.-S.; Cho, J. A highly cross-linked polymeric binder for high-performance silicon negative electrodes in lithium ion batteries. Angew. Chem. Int. Ed. 2012, 51, 8762–8767. [Google Scholar] [CrossRef]
- Mazouzi, D.; Karkar, Z.; Hernandez, C.R.; Manero, P.J.; Guyomard, D.; Roué, L.; Lestriez, B. Critical roles of binders and formulation at multiscales of silicon-based composite electrodes. J. Power Sources 2015, 280, 533–549. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-Ion battery Si-Based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Key, B.; Bhattacharyya, R.; Morcrette, M.; Seznéc, V.; Tarascon, J.-M.; Grey, C. Real-time NMR investigations of structural changes in silicon electrodes for Lithium-Ion batteries. J. Am. Chem. Soc. 2009, 131, 9239–9249. [Google Scholar] [CrossRef]
- Madelung, O.; Rössler, U.; Schulz, M. Indium antimonide (InSb), carrier concentrations: Datasheet from Landolt-Börnstein—Group III Condensed Matter Volume 41A1β. In Group IV Elements, IV-IV and III-V Compounds. Part b-Electronic, Transport, Optical and Other Properties; Springer: Berlin, Germany, 2002; pp. 1–6. [Google Scholar] [CrossRef]
- Isaacson, R.A. Electron spin resonance in n-Type InSb. Phys. Rev. 1968, 169, 312–314. [Google Scholar] [CrossRef]
- Orlov, V.G.; Sergeev, G. Numerical simulation of the transport properties of indium antimonide. Phys. Solid State 2013, 55, 2215–2222. [Google Scholar] [CrossRef]
- Agubra, V.; Fergus, J. Lithium ion battery anode aging mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef] [Green Version]
- Joho, F.; Rykart, B.; Blome, A.; Novák, P.; Wilhelm, H.; Spahr, M.E. Relation between surface properties, pore structure and first-cycle charge loss of graphite as negative electrode in lithium-ion batteries. J. Power Sources 2001, 97, 78–82. [Google Scholar] [CrossRef]
- Tran, T.D.; Feikert, J.H.; Pekala, R.W.; Kinoshita, K. Rate effect on lithium-ion graphite electrode performance. J. Appl. Electrochem. 1996, 26, 1161–1167. [Google Scholar] [CrossRef]
- Bläubaum, L.; Röder, F.; Nowak, C.; Chan, H.S.; Kwade, A.; Krewer, U. Impact of particle size distribution on performance of Lithium-Ion batteries. ChemElectroChem 2020, 7, 4755–4766. [Google Scholar] [CrossRef]
- Roselin, L.S.; Juang, R.-S.; Hsieh, C.-T.; Sagadevan, S.; Umar, A.; Selvin, R.; Hegazy, H.H. Recent advances and perspectives of carbon-based nanostructures as anode materials for Li-ion batteries. Materials 2019, 12, 1229. [Google Scholar] [CrossRef] [Green Version]
- Röder, F.; Braatz, R.D.; Krewer, U. Multi-scale simulation of heterogeneous surface film growth mechanisms in Lithium-Ion batteries. J. Electrochem. Soc. 2017, 164, E3335–E3344. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Yang, S.; Zhang, Z.; Lee, J.-M.; Zapien, J.A. Enhanced electrochemical performance of lithium ion batteries using Sb2S3 nanorods wrapped in graphene nanosheets as anode materials. Nanoscale 2018, 10, 3159–3165. [Google Scholar] [CrossRef]
- Wang, X.; Hwang, J.-Y.; Myung, S.-T.; Hassoun, J.; Sun, Y.-K. Graphene decorated by indium sulfide nanoparticles as high-performance anode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 23723–23730. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V.; Nam, K.-H.; Park, C.-M. Robust polyhedral CoTe2–C nanocomposites as high-performance Li- and Na-Ion battery anodes. ACS Appl. Energy Mater. 2020, 3, 4877–4887. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Shi, Q.; Xin, S.; Wu, J.; Zhang, C.-L.; Qiu, L.; Zhang, C. Facile synthesis of carbon-coated porous Sb2Te3 nanoplates with high alkali metal ion storage. ACS Appl. Mater. Interfaces 2019, 11, 29934–29940. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Tang, Y.; Li, W.; Yang, X.; Xue, H.; Yang, Q.; Lee, C.S. High interfacial storage capability of porous NiMn2O4/C hierarchical tremella-like nanostructures as the Lithium-Ion battery anode. Nanoscale 2015, 7, 225–231. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, H.; Lv, P.; Zhang, Z.; Wang, J.; Xia, Q. Electrochemical properties of iron oxides/carbon nanotubes as anode material for lithium ion batteries. J. Power Sources 2015, 274, 1091–1099. [Google Scholar] [CrossRef]
- Zhu, X.J.; Guo, Z.P.; Zhang, P.; Du, G.D.; Zeng, R.; Chen, Z.X.; Li, S.; Liu, H.K. Highly porous reticular tin–cobalt oxide composite thin film anodes for lithium ion batteries. J. Mater. Chem. 2009, 19, 8360–8365. [Google Scholar] [CrossRef]
- Fransson, L.; Vaughey, J.; Benedek, R.; Edström, K.; Thomas, J.; Thackeray, M. Phase transitions in lithiated Cu2Sb anodes for lithium batteries: An in situ X-ray diffraction study. Electrochem. Commun. 2001, 3, 317–323. [Google Scholar] [CrossRef]
- Baggetto, L.; Allcorn, E.; Unocic, R.R.; Manthiram, A.; Veith, G.M. Mo3Sb7 as a very fast anode material for lithium-ion and sodium-ion batteries. J. Mater. Chem. A 2013, 1, 11163–11169. [Google Scholar] [CrossRef]
- Wang, S.; He, M.; Walter, M.; Kravchyk, K.V.; Kovalenko, M.V. Monodisperse CoSb nanocrystals as high-performance anode material for Li-ion batteries. Chem. Commun. 2020, 56, 13872–13875. [Google Scholar] [CrossRef]
- Pan, Q.; Wu, Y.; Zhong, W.; Zheng, F.; Li, Y.; Liu, Y.; Hu, J.; Yang, C. Carbon nanosheets encapsulated NiSb nanoparticles as advanced anode materials for Lithium-Ion batteries. Energy Environ. Mater. 2020, 3, 186–191. [Google Scholar] [CrossRef]
- Hou, H.; Cao, X.; Yang, Y.; Fang, L.; Pan, C.; Yang, X.; Song, W.; Ji, X. NiSb alloy hollow nanospheres as anode materials for rechargeable lithium ion batteries. Chem. Commun. 2014, 50, 8201–8203. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Choi, S.; Hwang, C.; Ryu, J.; Song, W.-J.; Song, H.-K.; Park, S. Rational structure design of fast-charging NiSb Bimetal nanosheet anode for Lithium-ion batteries. Energy Fuels 2020, 34, 10211–10217. [Google Scholar] [CrossRef]
- Ning, X.; Li, Z. Centrifugally spun SnSb nanoparticle/porous carbon fiber composite as high-performance lithium-ion battery anode. Mater. Lett. 2021, 287, 129298. [Google Scholar] [CrossRef]
- Fan, L.; Liu, Y.; Tamirat, A.G.; Wang, Y.; Xia, Y. Synthesis of ZnSb@C microflower composites and their enhanced electrochemical performance for lithium-ion and sodium-ion batteries. New J. Chem. 2017, 41, 13060–13066. [Google Scholar] [CrossRef]
- Gómez-Cámer, J.L.; Novák, P. Polyacrylate bound TiSb2 electrodes for Li-ion batteries. J. Power Sources 2015, 273, 174–179. [Google Scholar] [CrossRef]








| Anode | Cycling Performance | Rate Capability | Synthesis Method | Ref. |
|---|---|---|---|---|
| Cu2Sb | 290 mAh·g−1 after 25 cycles | - | Ball milling | [60] |
| Mo3Sb7 | 350 mAh·g−1 after 100 cycles at 0.12 C | 300 mAh·g−1 at 100 C | Furnace | [61] |
| CoSb | 448 mAh·g−1 after 1000 cycles at 0.66 A·g−1 | - | Facile colloidal synthesis | [62] |
| NiSb@C | 405 mAh·g−1 after 1000 cycles at 0.1 A·g−1 | 393 mAh·g−1 at 2.0 A·g−1 | Freezing drying | [63] |
| NiSb hollow nanosphere | 420 mAh·g−1 after 50 cycles at 0.1 A·g−1 | 352 mAh·g−1 at 0.8 A·g−1 | Galvanic replacement reaction | [64] |
| NiSb/C nanosheet | 393 mAh·g−1 after 1000 cycles at 2 C | 325 mAh·g−1 at 10 C | Hydrothermal low-temperature carbothermic reduction | [65] |
| SnSb@Carbon fiber | 674 mAh·g−1 after 100 cycles at 0.1 A·g−1 | 163 mAh·g−1 at 1.6 A·g−1 | Electrospinning | [66] |
| ZnSb/C | 481 mAh·g−1 after 240 cycles at 0.1 A·g−1 | 426 mAh·g−1 at 0.5 A·g−1 | Annealing | [67] |
| TiSb2 | 420 mAh·g−1 after 120 cycles at 1 C | 300 mAh·g−1 at 12 C | Furnace | [68] |
| InSb_PAA InSb–C_PAA | 640 mAh·g−1 after 140 cycles 846 mAh·g−1 after 150 cycles at 0.1 A·g−1 | 594 mAh·g−1 at 10 A·g−1 716 mAh·g−1 at 10 A·g−1 | Ball milling | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang Huy, V.P.; Kim, I.T.; Hur, J. The Effects of the Binder and Buffering Matrix on InSb-Based Anodes for High-Performance Rechargeable Li-Ion Batteries. Nanomaterials 2021, 11, 3420. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123420
Hoang Huy VP, Kim IT, Hur J. The Effects of the Binder and Buffering Matrix on InSb-Based Anodes for High-Performance Rechargeable Li-Ion Batteries. Nanomaterials. 2021; 11(12):3420. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123420
Chicago/Turabian StyleHoang Huy, Vo Pham, Il Tae Kim, and Jaehyun Hur. 2021. "The Effects of the Binder and Buffering Matrix on InSb-Based Anodes for High-Performance Rechargeable Li-Ion Batteries" Nanomaterials 11, no. 12: 3420. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11123420