Graphene Quantum Dots by Eco-Friendly Green Synthesis for Electrochemical Sensing: Recent Advances and Future Perspectives
Abstract
:1. Introduction
2. GQDs from Eco-Friendly Raw Materials by Green Approaches
2.1. Oxidative Method
2.2. Laser Ablation
2.3. Controllable Synthesis
2.4. Pyrolysis
2.5. Hydrothermal Method
2.6. Microwave Irradiation Method
3. GQDs Obtained by Ecofriendly Synthesis for Electrochemical Sensors
3.1. GQDs Electrochemical Sensors for Neurotransmitter Detection
3.2. Electrochemical Sensors for Biomarkers Detection
3.3. GQDs Based Electrochemical Sensors for Environmental Monitoring
3.4. GQDs Based Electrochemical Sensors for Food Analysis
4. Challenges and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sheldon, R.A. Green chemistry and resource efficiency: Towards a green economy. Green Chem. 2016, 18, 3180–3183. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-derived carbon: Synthesis and applications in energy storage and conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Li, N.; Than, A.; Wang, X.W.; Xu, S.H.; Sun, L.; Duan, H.W.; Xu, C.J.; Chen, P. Ultrasensitive profiling of metabolites using tyramine-functionalized graphene quantum dots. ACS Nano. 2016, 10, 3622–3629. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, Y.; Li, N.; Li, W.; Wang, Z.; Zhu, J.; Zhang, H.; Liu, B.; Xu, S. Synthesis of luminescent graphene quantum dots with high quantum yield and their toxicity study. PLoS ONE 2015, 10, e0144906. [Google Scholar] [CrossRef]
- Wang, S.; Cole, I.S.; Li, Q. The toxicity of graphene quantum dots. RSC Adv. 2016, 6, 89867–89878. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.; Tuteja, S.; Vinayak, P.; Paul, A.; Kim, K.; Deep, A. Graphene quantum dot modified screen printed immunosensor for the determination of parathion. Anal. Biochem. 2017, 523, 1–9. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Graphene Quantum Dot-Based Electrochemical Immunosensors for Biomedical Applications. Materials 2020, 13, 96. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, W.; Wu, B.; Li, Z.; Wang, S.; Liu, Y.; Pan, D.; Wu, M. Facile synthesis of fluorescent graphene quantum dots from coffee grounds for bioimaging and sensing. Chem. Eng. J. 2016, 300, 75–82. [Google Scholar] [CrossRef]
- Lu, H.; Li, W.; Dong, H.; Wei, M. Graphene Quantum Dots for Optical Bioimaging. Small 2019, 15, 1902136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.R.; Deshmukh, K.; Sadasivuni, K.K.; Pasha, S.K.K. Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: A review. RSC Adv. 2020, 10, 23861–23898. [Google Scholar] [CrossRef]
- Ghosh, D.; Kapri, S.; Bhattacharyya, S. Phenomenal Ultraviolet Photoresponsivity and Detectivity of Graphene Dots Immobilized on Zinc Oxide Nanorods. ACS Appl. Mater. Interfaces 2016, 8, 35496–35504. [Google Scholar] [CrossRef]
- Ghosh, D.; Sarkar, K.; Devi, P.; Kim, K.H.; Kumar, P. Current and future perspectives of carbon and graphene quantum dots: From synthesis to strategy for building optoelectronic and energy devices. Renew. Sustain. Energy Rev. 2021, 135, 110391. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Yuan, H.; Dong, X.; Zhang, Y.; Asif, M.; Dong, Z.; He, W.; Ren, J.; Sun, Y.; Xiao, F. Dual nanoenzyme modified microelectrode based on carbon fiber coated with AuPd alloy nanoparticles decorated graphene quantum dots assembly for electrochemical detection in clinic cancer samples. Biosens. Bioelectron. 2018, 107, 153–162. [Google Scholar] [CrossRef]
- Islam, M.S.; Deng, Y.; Tong, L.; Roy, A.K.; Faisal, S.N.; Hassan, M.; Minett, A.I.; Gomes, V.G. In-situ direct grafting of graphene quantum dots onto carbon fibre by low temperature chemical synthesis for high performance flexible fabric supercapacitor. Mater. Today Commun. 2017, 10, 112–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Chen, N.; Qu, L. Graphene quantum dots: An emerging material for energy-related applications and beyond. Energy Environ. Sci. 2012, 5, 8869–8890. [Google Scholar] [CrossRef]
- Zhao, J.; Tang, L.; Xiang, J.; Ji, R.; Hu, Y.; Yuan, J.; Zhao, J.; Tai, Y.; Cai, Y. Fabrication and properties of a high-performance chlorine doped graphene quantum dot based photo-voltaic detector. RSC Adv. 2015, 5, 29222–29229. [Google Scholar] [CrossRef]
- Shojaei, T.R.; Mohd Salleh, M.A.; Mobli, H.; Aghbashlo, M.; Tabatabei, M. Multivariable optimization of carbon nanoparticles synthesized from waste facial tissues by artificial neural networks, new material from downstream quenching of quantum dots. J. Mater. Sci. Mater. Electron. 2019, 30, 3156–3165. [Google Scholar] [CrossRef]
- Pistone, A.; Espro, C. Current trends on turning biomass wastes into carbon materials for electrochemical sensing and rechargeable battery applications. Curr. Opin. Green Sustain. Chem. 2020, 26, 100374. [Google Scholar] [CrossRef]
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental challenges impeding the composting of biodegradable municipal solid waste: A critical review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Abbas, A.; Mariana, L.T.; Phana, A.N. Biomass-waste derived graphene quantum dots and their applications. Carbon 2018, 140, 77–99. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Li, F.; Wen, J.; Wang, X.; Sun, R. Gram-scale synthesis of single-crystalline graphene quantum dots derived from lignin biomass. Green Chem. 2018, 20, 1383–1390. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Yaxuan, J.; Guo, Y.; Qineng, X.; Xiaohui, L.; Wang, Y. Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellulosic Biomass. Chem 2019, 5, 2520–2546. [Google Scholar]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef]
- Nirala, N.R.; Khandelwal, G.; Kumar, B.; Vinita; Prakash, R.; Kumar, V. One step electro-oxidative preparation of graphene quantum dots from wood charcoal as a peroxidase mimetic. Talanta 2017, 173, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, J.; Zhang, X.; Li, N.; Liu, B.; Li, Y.; .Wang, Y.; Wang, W.; Li, Y.; Zhang, L.; et al. Large-scale and controllable synthesis of graphene quantum dots from rice husk bio-mass: A comprehensive utilization strategy. ACS Appl. Mater. 2016, 8, 1434. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Sudolska, M.; Kalytchuk, S.; Nachtigallova, D.; Rogach, A.; Otyepka, M.; Zboril, R. Graphitic nitrogen triggers red fluorescence in carbon dots. ACS Nano 2017, 11, 12402. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew. Chem. Int. 2013, 52, 3953. [Google Scholar] [CrossRef]
- Mahesh, S.; Lekshmi, C.L.; Renuka, K.D.; Joseph, K. Simple and Cost-Effective Synthesis Fluorescent Graphene Quantum Dots from Honey: Application as Stable Security Ink and White-Light Emission. Particle 2016, 33, 70–74. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene Quantum Dots from Mangifera indica: Application in NearInfrared Bioimaging and Intracellular Nanothermometry. ACS Sustain. Chem. Eng. 2017, 5, 1382–1391. [Google Scholar] [CrossRef]
- Dager, A.; Baliyan, A.; Kurosu, S.; Maekawa, T.; Tachibana, M. Ultrafast synthesis of carbon quantum dots from fenugreek seeds using microwave plasma enhanced decomposition: Application of C-QDs to grow fluorescent protein crystals. Sci. Rep. 2020, 10, 12333. [Google Scholar] [CrossRef]
- Kulchitsky, V.A.; Davydov, M.; Osipov, A.N.; Kilin, S.Y. Neural network Structures:Current and Future States. OSTIS 2018, 1, 259–264. [Google Scholar]
- Liu, Q.; Zhang, J.; He, H.; Huang, G.; Xing, B.; Jia, J.; Zhang, C. Green preparation of high yield fluorescent graphene quantum dots from coal-tar-pitch by mild oxidation. Nanomaterials 2018, 8, 844. [Google Scholar] [CrossRef] [Green Version]
- Halder, A.; Godoy-Gallardo, M.; Ashley, J.; Feng, X.; Zhou, T.; Hosta-Rigau, L.; Sun, Y. One-Pot Green Synthesis of Biocompatible Graphene Quantum Dots and Their Cell Uptake Studies. ACS Appl. Bio. Mater. 2018, 1, 452–461. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green synthesis, biomedical and biotechnological applications of carbon and graphene quantum dots. A review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Zhang, X.; Tong, X.; Wang, X.; Yang, P.; Yao, F.; Guo, R.; Yuan, C. Preparation of graphene quantum dots with high quantum yield by a facile one-step method and applications for cell imaging. Mater. Lett. 2020, 271, 127806. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- He, M.; Guo, X.; Huang, J.; Shen, H.; Zeng, Q.; Wang, L. Mass production of tunable multicolor graphene quantum dots from an energy resource of coke by a one-step electrochemical exfoliation. Carbon 2018, 140, 508–520. [Google Scholar] [CrossRef]
- Duarte de Menezes, F.; dos Reis, S.R.R.; Pinto, S.R.; Portilho, F.L.; Chaves e Mello, F.; Helal-Neto, E.; da Silva de Barros, A.O.; Alencar, L.M.R.; Silva de Menez, A.; Costa dos Santos, C.; et al. Graphene quantum dots unraveling: Green synthesis, characterization, radiolabeling with 99 mTc, in vivo behavior and mutagenicity. Mater. Sci. Eng. 2019, 102, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.K.; Lakshmi, S.B.; Santra, T.S.; Ramachandra Rao, M.S.; Krishnamurthi, G. Oxygenated graphene quantum dots (GQDs) synthesized using laser ablation for long-term real-time tracking and imaging. RSC Adv. 2017, 7, 53822–53829. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Jeong, Y.K.; Jung, K.H.; Son, Y.; Kim, W.R.; Ryu, J.H.; Kim, K.M. One-step synthesis of sulfur-incorporated graphene quantum dots using pulsed laser ablation for enhancing optical properties. Opt. Express 2020, 28, 21659–21667. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Hu, W.; Li, D.; Chen, S.; Dai, Z. Synthesis and applications of graphene quantum dots: A review. Nanotech. Rev. 2018, 7, 157–185. [Google Scholar] [CrossRef]
- Yan, X.; Cui, X.; Li, L. Synthesis of Large, Stable Colloidal Graphene Quantum Dots with Tunable Size. J. Am. Chem. Soc. 2010, 132, 5944–5945. [Google Scholar] [CrossRef]
- Lu, Y.; Hao, H.; Liu, P.; Feng, Y.; Wang, J. Controllable synthesis of Graphene Quantum Dots with Tunable-Photoluminescence. Mater. Sci. Eng. 2020, 768. [Google Scholar] [CrossRef]
- Naik, J.P.; Sutradhar, P.; Saha, M. Molecular scale rapid synthesis of graphene quantum dots (GQDs). J. Nanostruct. Chem. 2017, 7, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh, J.; Khataee, A. Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta 2018, 178, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Veeramani, V.; Sivakumar, M.; Chen, S.M.; Madhu, R.; Alamri, H.R.; Alothman, Z.A.; Hossain, S.A.; Chen, C.K.; Yamauchi, Y.; Miyamoto, N.; et al. Lignocellulosic biomass-derived, graphene sheet-like porous activated carbon for electrochemical supercapacitor and catechin sensing. RSC Adv. 2017, 7, 45668–45675. [Google Scholar] [CrossRef] [Green Version]
- Kalita, H.; Mohapatra, J.; Pradhanb, L.; Mitraa, A.; Bahadurc, D.; Aslam, M. Efficient synthesis of rice based graphene quantum dots and their fluorescent properties. RSC Adv. 2016, 6, 23518–23524. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Z.; Liu, J.; Peng, Y.; Yu, X.; Wang, W. One-Pot Facile Synthesis of Graphene Quantum Dots from Rice Husks for Fe3+ Sensing. Ind. Eng. Chem. Res. 2018, 57, 9144–9150. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.; Zheng, X.; Xu, A.; Yang, S. Facile and Highly Effective Synthesis of Controllable Lattice Sulfur-Doped Graphene Quantum Dots via Hydrothermal Treatment of Durian. ACS Appl. Mater. Interfaces 2018, 10, 5750–5759. [Google Scholar] [CrossRef]
- Tade, R.S.; Patil, P.O. Green synthesis of fluorescent graphene quantum dots and its application in selective curcumin detection. Curr. Appl. Phys. 2020, 20, 1226–1236. [Google Scholar] [CrossRef]
- Eom, Y.; Min Son, S.; Kim, Y.E.; Lee, J.E.; Hwang, S.; Cha, H.G. Structure evolution mechanism of highly ordered graphite during carbonization of cellulose nanocrystals. Carbon 2019, 150, 142–152. [Google Scholar] [CrossRef]
- Ahmed, D.S.; Mohammed, M.; Majeed, S.M. Green Synthesis of Eco-Friendly Graphene Quantum Dots for Highly Efficient Perovskite Solar Cells. ACS Appl. Energy Mater. 2020, 3, 10863–10871. [Google Scholar] [CrossRef]
- Foong, L.K.; Khojasteh, H.; Amiri, M.; Heydaryan, K.; Salavati-Niasari, M.; Almasi-Kashi, M.; Lyu, Z. Environmental friendly approach for facile synthesis of graphene-like nanosheets for photocatalytic activity. J. Alloys Compd. 2020, 823, 153696. [Google Scholar] [CrossRef]
- Bayat, A.; Saievar-Iranizad, E. Synthesis of green-photoluminescent single layer graphene quantum dots: Determination of HOMO and LUMO energy states. J. Lumin. 2017, 192, 180–183. [Google Scholar] [CrossRef]
- Chen, W.; Shen, J.; Lv, G.; Li, D.; Hu, Y.; Zhou, C.; Liu, X.; Dai, Z. Green Synthesis of Graphene Quantum Dots from Cotton Cellulose. Chem. Sel. 2019, 4, 2898–2902. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Tian, L.; Xiang, W. Green synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- Safaei-Ghomi, J.; Elyasi, Z.; Babaei, P. N-doped graphene quantum dots modified with CuO (0D)/ZnO (1D) heterojunctions as a new nanocatalyst for the environmentally friendly one-pot synthesis of monospiro derivatives. New J. Chem. 2021, 45, 1269–1277. [Google Scholar] [CrossRef]
- Zhu, Q.; Mao, H.; Li, J.; Hua, J.; Wang, J.; Yang, R.; Li, Z. A glycine-functionalized graphene quantum dots synthesized by a facile post-modification strategy for a sensitive and selective fluorescence sensor of mercury ions. Spectrochim Acta A Mol. Biomol. Spectrosc. 2021, 247, 119090. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Hashemzadeh, N.; Shadjou, N.; Eivazi-Ziaei, J.; Khoubnasabjafari, M.; Jouyban, A. Sensing of doxorubicin hydrochloride using graphene quantum dot modified glassy carbon electrode. J. Mol. Liq. 2016, 221, 354–357. [Google Scholar] [CrossRef]
- Qu, Z.; Na, W.; Liu, X.; Liu, H.; Su, X. A novel fluorescence biosensor for sensitivity detection of tyrosinase and acid phosphatase based on nitrogen-doped graphene quantum dots. Anal. Chim. Acta 2018, 997, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Mao, W.; Huang, J.; Li, S.; Huang, K.; Li, M.; Xia, J.; Chen, Q. Economical, green route to highly fluorescence intensity carbon materials based on ligninsulfonate/graphene quantum dots composites: Application as excellent fluorescent sensing platform for detection of Fe3+ ions. Sens. Actuators B Chem. 2016, 230, 54–60. [Google Scholar] [CrossRef]
- Singh, R.K.; Kumar, R.; Singh, D.P.; Savu, R.; Moshkalev, S.A. Progress in microwave-assisted synthesis of quantum dots (graphene/carbon/semiconducting) for bioapplications: A review. Mater. Today Chem. 2019, 12, 282–314. [Google Scholar] [CrossRef]
- Abbas, A.; Tabish, T.A.; Bull, S.J.; Lim, T.M.; Phan, A.N. High yield synthesis of graphene quantum dots from biomass waste as a highly selective probe for Fe3+ sensing. Sci. Rep. 2020, 10, 21262. [Google Scholar] [CrossRef]
- Tak, K.; Sharma, R.; Dave, V.; Jain, S.; Sharma, S. Clitoria ternatea Mediated Synthesis of Graphene Quantum Dots for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2020, 11, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.X.; Chiang, W.H. Microplasma-assisted synthesis of silver nanoparticle/graphene quantum dots nanohybrids for photoluminescence-based silver ion and biothiols detection. ACS Appl. Mater. Interfaces 2020, 12, 28550–28560. [Google Scholar]
- Thakur, M.; Mewada, A.; Pandey, S.; Bhori, M.; Singh, K.; Sharon, M.; Sharon, M. Milk-derived multi-fluorescent graphene quantum dot-based cancertheranostic system. Mater. Sci. Eng. C 2016, 67, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shao, F.Q.; Huang, H.; Feng, J.J.; Wang, A.J. Eco-friendly and rapid microwave synthesis of green fluorescent graphitic carbon nitride quantum dots for vitro bioimaging. Sens. Actuators B Chem. 2016, 226, 506–511. [Google Scholar] [CrossRef]
- Kumawat, M.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene Quantum Dots for Cell Proliferation, Nucleus Imaging, and Photoluminescent Sensing Applications. Sci. Rep. 2017, 7, 15858. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Haque, E.; Kim, J.; Malgras, V.; Reddy, K.R.; Ward, A.C.; You, J.; Bando, Y.; Hossain, S.A.; Yamauchi, Y. Recent Advances in Graphene Quantum Dots: Synthesis, Properties, and Applications. Small Methods 2018, 2, 1800050. [Google Scholar] [CrossRef]
- Kadian, S.; Sethi, S.K.; Manik, G. Recent advancements in synthesis and property control of graphene quantum dots for biomedical and optoelectronic applications. Mater. Chem. Front. 2021, 5, 627–658. [Google Scholar] [CrossRef]
- Choi, S. Unique properties of graphene quantum dots and their applications in photonic/electronic devices. J. Phys. D Appl. Phys. 2017, 50, 103002–103012. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Faridbod, F.; Sanati, A.L. Graphene Quantum Dots in Electrochemical Sensors/Biosensors. Curr. Anal. Chem. 2019, 15, 103–123. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. Synthesis of highly fluorescent nitrogen-doped graphene quantum dots for sensitive, label-free detection of Fe (III) in aqueous media. Biosens. Bioelectron. 2014, 58, 219–225. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. Graphene quantum dots as fluorescence probes for sensing metal ions: Synthesis and applications. Curr. Org. Chem. 2015, 19, 1150–1162. [Google Scholar] [CrossRef]
- Fan, L.; Hu, Y.; Wang, X. Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT. Talanta 2012, 101, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, W. In situ growth of surfactant-free gold nanoparticles on nitrogen-doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar] [CrossRef]
- Shehabab, M.; Ebrahima, S.; Solimana, M. Graphene quantum dots prepared from glucose as optical sensor for glucose. J. Lumin. 2017, 184, 110–116. [Google Scholar] [CrossRef]
- Nyokong, T.; Achadu, O. Graphene quantum dots decorated with maleimide and zinc tetramaleimido-phthalocyanine: Application in the design of “OFF-ON” fluorescence sensors for biothiols. Talanta 2017, 166, 15–26. [Google Scholar]
- Fei, X.; Liu, Z.; Li, Y.; Yang, G.; Su, C.; Zhong, H.; Zhuang, Z.; Guo, Z. One-pot green synthesis of flower-liked Au NP@GQDs nanocomposites for surface-enhanced Raman scattering. J. Alloys Compd. 2017, 725, 1084–1090. [Google Scholar] [CrossRef]
- Dong, P.; Jiang, B.; Liang, W.; Huang, Y.; Shi, Z.; Shen, X. Synthesis of white-light-emitting graphene quantum dots via a one-step reduction and their interfacial characteristics-dependent luminescence properties. Inorg. Chem. Front. 2017, 4, 71. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Salamo, M.; Galvagno, S.; Romeo, R.; Giofre, S.V.; Branca, C.; Visalli, G.; Di Pietro, A. Graphene quantum dots for cancer targeted drug delivery. Int. J. Pharm. 2017, 518, 185–192. [Google Scholar] [CrossRef]
- Jegannathan, P.; Yousefi, A.; Kadri, N.; Basirun, W. Sustainable GQDs for potential application in engineering using corn powder as green precursor. Fuller. Nanot. Carb. Nanostr. 2020, 28, 919–924. [Google Scholar] [CrossRef]
- Tomkins, D.M.; Sellers, E.M. Addiction and the brain: The role of neurotransmitters in the cause and treatment of drug dependence. Can. Med. Assoc. J. 2001, 164, 817. [Google Scholar]
- Sitruk-Ware, R. Progestogens in hormonal replacement therapy: Newmolecules, risks, and benefits. Menopause 2002, 9, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.L.; Dishman, R.K. Exercise and the Neurobiology of Depression. Exerc. Sport Sci. Rev. 1991, 19, 41–98. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Karimzadeh, A.; Shadjou, A.; Mokhtarzadeh, A.; Bageri, L.; Sadeghi, S.; Mahboob, S. Graphene quantum dots decorated with magnetic nanoparticles: Synthesis, electrodeposition, characterization and application as an electrochemical sensor towards determination of some amino acids at physiological pH. Mater. Sci. Eng. 2016, 68, 814. [Google Scholar] [CrossRef] [Green Version]
- Habibi, E.; Heidari, H. Renewable surface carbon-composite electrode bulk modified with GQD-RuCl3 nano-composite for high sensitive detection of l-tyrosine. Electroanalysis 2016, 28, 2559. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M.; Talebi, F. Graphene quantum dots incorporated into b-cyclodextrin: A novel polymeric nanocomposite for non-enzymatic sensing of Ltyrosine at physiological pH. J. Anal. Chem. 2018, 73, 602. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Y.; Mo, T.; Zhou, H.; Li, Y.; Li, S. Highly selective dopamine sensor based on graphene quantum dots self-assembled monolayers modified electrode. J. Electroanal. Chem. 2016, 767, 1–194. [Google Scholar] [CrossRef]
- Ben Aoun, S. Nanostructured carbon electrode modified with N-doped graphene quantum dots-chitosan nanocomposite: A sensitive electrochemical dopamine sensor. R. Soc. Open Sci. 2017, 4, 171199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashkhourian, J.; Nami-Ana, S.; Shamsipur, M. Designing a modified electrode based on graphene quantum dot-chitosan application to electrochemical detection of epinephrine. J. Mol. Liq. 2018, 266, 548–556. [Google Scholar] [CrossRef]
- Ruiyi, L.; Sili, Q.; Zhangyi, L.; Ling, L.; Zaijun, L. Histidine-functionalized graphene quantum dot-graphene micro-aerogel based voltammetric sensing of dopamine. Sens. Actuators B Chem. 2017, 250, 372–382. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, R.; Ma, X.; Tang, J.; Li, Z.; Wang, X.; Wang, J. A highly sensitive dopamine sensor based on graphene quantum dots modified glassy carbon electrode. Int. J. Electrochem. Sci. 2018, 13, 5723. [Google Scholar] [CrossRef]
- Arumugasamy, S.; Govindaraju, S.; Yun, K. Electrochemical sensor for detecting dopamine using graphene quantum dots incorporated with multiwall carbon nanotubes. Appl. Surf. Sci. 2020, 508, 145294. [Google Scholar] [CrossRef]
- Vinoth, V.; Natarajan, L.; Mangalaraja, R.; Valdes, H.; Anandan, S. Simultaneous electrochemical determination of dopamine and epinephrine using gold nanocrystals capped with graphene quantum dots in a silica network. Microchim. Acta 2019, 186, 68. [Google Scholar] [CrossRef]
- Baluta, S.; Lesiak, A.; Cabaj, J. Graphene quantum dots-based electrochemical biosensor for catecholamine neurotransmitters detection. Electroanalysis 2018, 30, 1781. [Google Scholar] [CrossRef]
- Fajardo, A.; Tapia, D.; Pizarro, J.; Segura, R.; Jara, P. Determination of norepinephrine using a glassy carbon electrode modified with graphene quantum dots and gold nanoparticles by square wave stripping voltammetry. J. Appl. Electrochem. 2019, 49, 423. [Google Scholar] [CrossRef]
- Arvand, M.; Hemmati, S. Analytical methodology for the electro-catalytic determination of estradiol and progesterone based on graphene quantum dots and poly(sulfosalicylic acid) co-modified electrode. Talanta 2017, 174, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Hemmati, S. Magnetic nanoparticles embedded with graphene quantum dots and multiwalled carbon nanotubes as a sensing platform for electrochemical detection of progesterone. Sens. Actuators B Chem. 2017, 238, 346–356. [Google Scholar] [CrossRef]
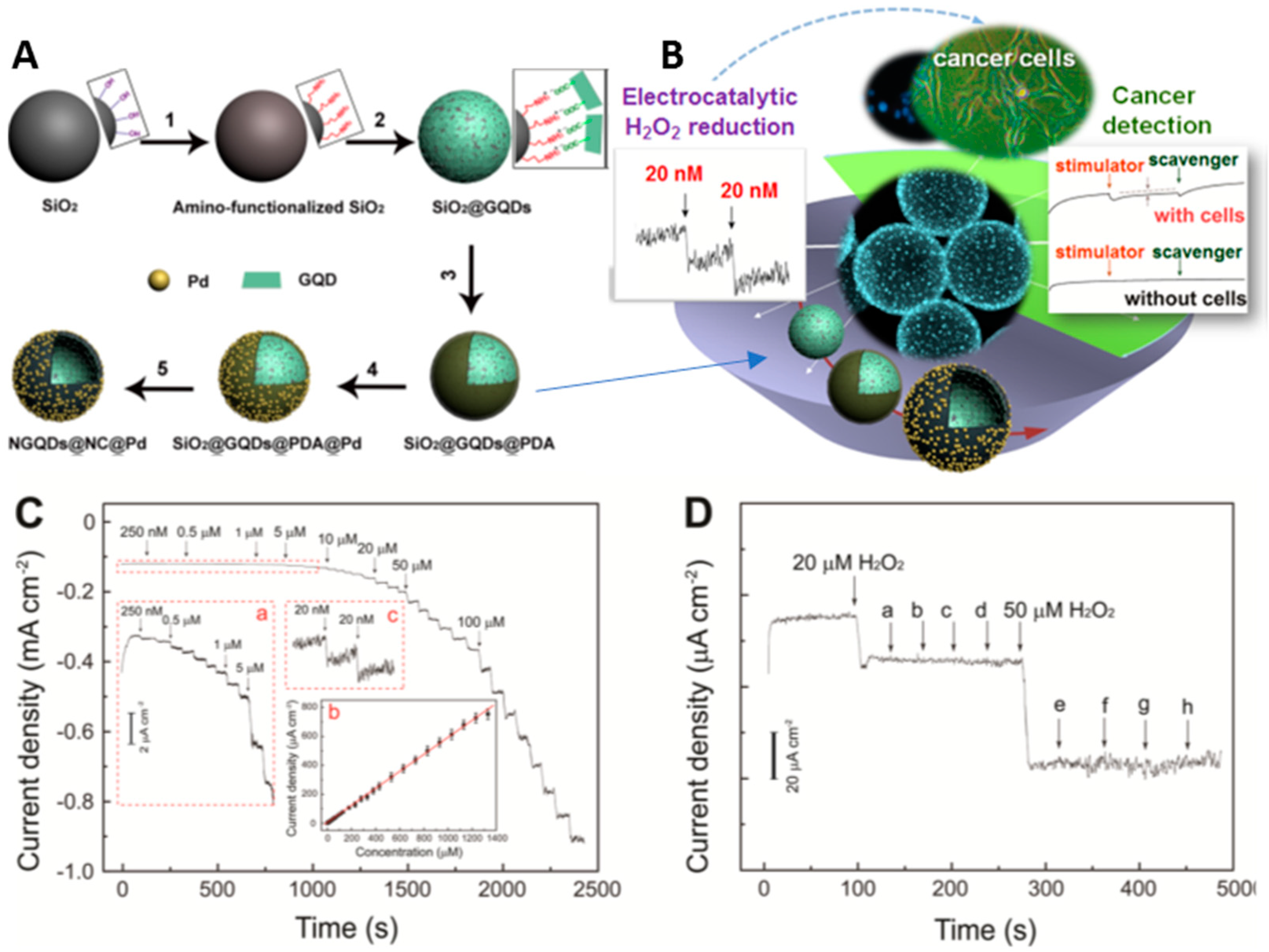
- Xi, J.; Xie, C.; Zhang, Y.; Wang, L.; Xiao, J.; Duan, X.; Ren, J.; Xiao, F.; Wang, S. Pd Nanoparticles Decorated N-Doped Graphene Quantum Dots@N-Doped carbon hollow nanospheres with high electrochemical sensing performance in cancer detection. ACS. Appl. Mater. Interf. 2016, 8, 22563–22573. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Li, S. A sensitive enzyme-free hydrogen peroxide sensor based on a chitosan-graphene quantum dot/silver nanocube nanocomposite modified electrode. Anal. Methods 2016, 8, 2448–2455. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Q.; Liu, Y.; Cui, J.; Liu, H.; Wang, P.; Li, Y.; Chen, L.; Zhao, Z.; Dong, Y. A novel label-free electrochemical immunosensor based on functionalized nitrogen-doped grapheme quantum dots for carcinoembryonic antigen detection. Biosens. Bioelectron. 2017, 90, 31–38. [Google Scholar] [CrossRef]
- Shadjou, N.; Hasanzadeh, M.; Talebi, F.; Marjani, A. Integration of β-cyclodextrin into graphene quantum dot nano-structure and its application towards detection of Vitamin C at physiological pH: A new electrochemical approach. Mater. Sci. Eng. C 2016, 67, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, J.; Yang, R.; Qu, L.; de Harrington, P.B. A sensitive and selective electrochemical sensor based on graphene quantum dot/gold nanoparticle nanocomposite modified electrode for the determination of quercetin in biological samples. Electroanal. 2016, 28, 1322–1330. [Google Scholar] [CrossRef]
- Xiang, Q.; Huang, J.; Huang, H.; Mao, W.; Ye, Z. A label-free electrochemical platform for the highly sensitive detection of hepatitis B virus DNA using graphene quantum dots. RSC Adv. 2018, 8, 1820–1825. [Google Scholar] [CrossRef] [Green Version]
- Jie, G.; Zhou, Q.; Jie, G. Graphene quantum dots-based electrochemiluminescence detection of DNA using multiple cycling amplification strategy. Talanta 2019, 194, 658–663. [Google Scholar] [CrossRef]
- Trinadh, T.; Khuntia, H.; Anusha, T.; Bhavani, K.; Kumar, J.; Brahman, P. Synthesis and characterization of nanocomposite material based on graphene quantum dots and lanthanum doped zirconia nanoparticles: An electrochemical sensing application towards flutamide in urine samples. Diam. Relat. Mater. 2020, 110, 108143. [Google Scholar] [CrossRef]
- Santos, A.; Wong, A.; Prado, T.; Fava, E.; Fatibello-Filho, O.; Sotomayor, M.; Moraes, F. Voltammetric determination of ethinylestradiol using screen-printed electrode modified with functionalized graphene, graphene quantum dots and magnetic nanoparticles coated with molecularly imprinted polymers. Talanta 2021, 224, 121804. [Google Scholar] [CrossRef]
- Jian, X.; Liu, X.; Yang, H.; Guo, M.; Song, X.; Dai, H.; Liang, Z. Graphene quantum dots modified glassy carbon electrode via electrostatic self-assembly strategy and its application. Electrochim. Acta 2016, 190, 455–462. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, S.; Li, M.; Li, W.; Zhao, Y.; Qi, Y.; Cui, X. Graphene quantum dots decorated graphene as an enhanced sensing platform for sensitive and selective detection of copper(II). J. Electroanal. Chem. 2017, 797, 113–120. [Google Scholar] [CrossRef]
- Tian, B.; Kou, Y.; Jiang, .X.; Lu, J.; Xue, Y.; Wang, M.; Tan, L. Ultrasensitive determination of mercury ions using a glassy carbon electrode modified with nanocomposites consisting of conductive polymer and amino-functionalized graphene quantum dots. Microchim. Acta 2020, 187, 210. [Google Scholar] [CrossRef]
- Fu, C.; Hsieh, C.; Juang, R.; Gu, S.; Gandomi, Y.; Kelly, R.; Kihm, K. Electrochemical sensing of mercury ions in electrolyte solutions by nitrogen-doped graphene quantum dot electrodes at ultralow concentrations. J. Mol. Liq. 2020, 302, 112593. [Google Scholar] [CrossRef]
- Punrat, E.; Maksuk, C.; Chuanuwatanakul, S.; Wonsawat, W.; Chailapakul, O. Polyaniline/graphene quantum dot-modified screen-printed carbon electrode for the rapid determination of Cr(VI) using stopped-flow analysis coupled with voltammetric technique. Talanta 2016, 150, 198–205. [Google Scholar] [CrossRef] [PubMed]
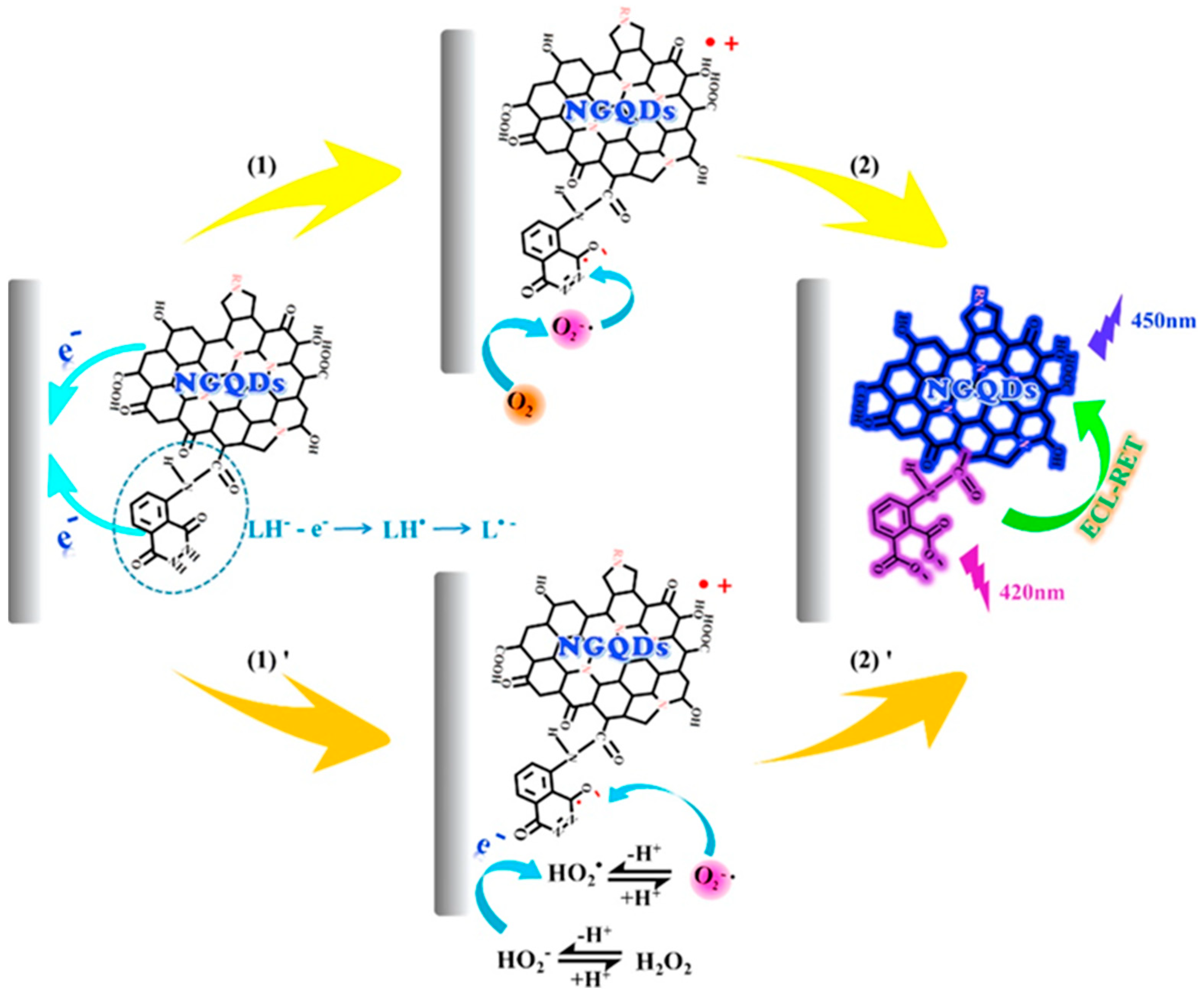
- Tian, K.; Li, D.; Tang, T.; Nie, F.; Zhou, Y.; Du, J.; Zheng, J. A novel electrochemiluminescence resonance energy transfer system of luminol-graphene quantum dot composite and its application in H2O2 detection. Talanta 2018, 185, 446–452. [Google Scholar] [CrossRef]
- Arvand, M.; Abbasnejad, S.; Ghodsi, N. Graphene quantum dots decorated with Fe3O4 nanoparticles/functionalized multiwalled carbon nanotubes as a new sensing platform for electrochemical determination of L-DOPA in agricultural products. Anal. Methods. 2016, 8, 5861–5868. [Google Scholar] [CrossRef]
- Wang, L.; Tricard, S.; Yue, P.; Zhao, J.; Fang, J.; Shen, W. Polypyrrole and graphene quantum dots @ Prussian Blue hybrid film on graphite felt electrodes: Application for amperometric determination of l-cysteine. Biosens. Bioelectron. 2016, 77, 1112–1118. [Google Scholar] [CrossRef]
- Ghiasi, T.; Ahmadi, S.; Ahmadi, E.; Olyai, M.; Khodadadi, Z. Novel electrochemical sensor based on modified glassy carbon electrode with graphene quantum dots, chitosan and nickel molybdate nanocomposites for diazinon and optimal design by the Taguchi method. Microchem. J. 2020, 160, 105628. [Google Scholar] [CrossRef]
- Shadjou, R.; Hasanzadeh, M.; Heidar-Poor, M.; Shadjou, N. Electrochemical monitoring of aflatoxin M1 in milk samples using silver nanoparticles dispersed on α-cyclodextrin-GQDs nanocomposite. J. Mol. Recognit. 2018, 31, 2699. [Google Scholar] [CrossRef]
- Gupta, P.; Chauhan, D.; Khan, Z.; Solanki, P. ZrO2 Nanoflowers Decorated with Graphene Quantum Dots for Electrochemical Immunosensing. ACS Appl. Nano Mater. 2020, 3, 2506–2516. [Google Scholar] [CrossRef]
- Sanati, A.; Faridbod, F.; Ganjali, M. Synergic effect of graphene quantum dots and room temperature ionic liquid for the fabrication of highly sensitive voltammetric sensor for levodopa determination in the presence of serotonin. J. Mol. Liq. 2017, 241, 316–320. [Google Scholar] [CrossRef]
- Kunpatee, K.; Traipop, S.; Chailapakul, O.; Chuanuwatanakul, S. Simultaneous determination of ascorbic acid, dopamine, and uric acid using graphene quantum dots/ionic liquid modified screen-printed carbon electrode. Sens. Actuators B Chem. 2020, 314, 128059. [Google Scholar] [CrossRef]
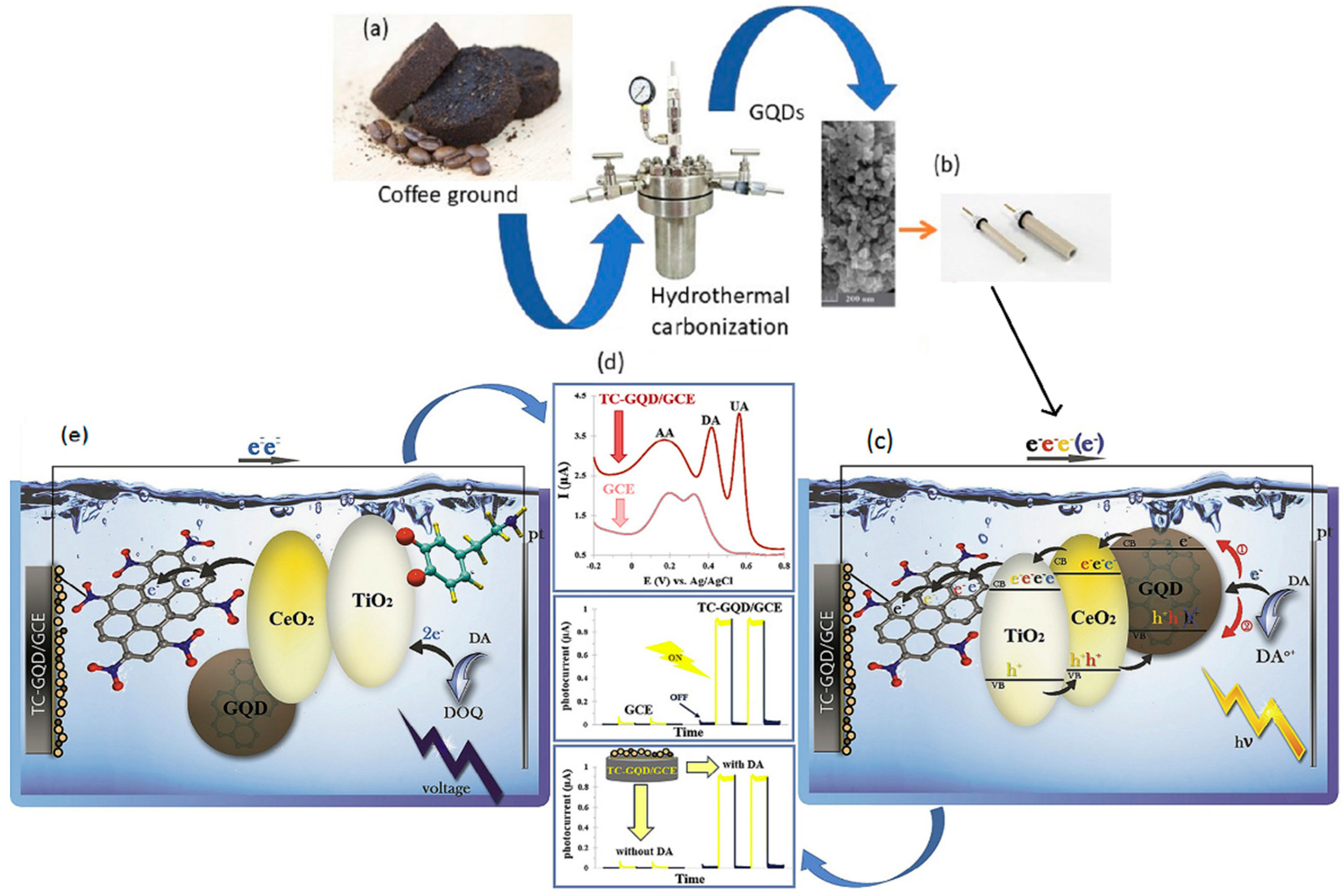
- Ahmadi, N.; Bagherzadeh, M.; Nemati, A. Comparison between electrochemical and photoelectrochemical detection of dopamine based on titania-ceria-graphene quantum dots nanocomposite. Biosens. Bioelectron. 2020, 151, 111977. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Hsieh, C.; Mallick, B.; Fu, C.; Juang, R.; Gandomi, Y.; Kihm, K. Non-enzymatic electrochemical detection of hydrogen peroxide on highly amidized graphene quantum dot electrodes. Appl. Surf. Sci. 2020, 528, 146936. [Google Scholar] [CrossRef]
- Xiaoyan, Z.; Ruiyi, L.; Zaijun, L.; Zhiguo, G.; Guangli, W. Ultrafast synthesis of gold/proline-functionalized graphene quantum dots and its use for ultrasensitive electrochemical detection of: P-acetamidophenol. RSC. Adv. 2016, 6, 42751–42755. [Google Scholar] [CrossRef]
- Samuei, S.; Fakkar, J.; Rezvani, Z.; Shomali, A.; Habibi, B. Synthesis and characterization of graphene quantum dots/CoNiAl-layered double-hydroxide nanocomposite: Application as a glucose sensor. Anal. Biochem. 2017, 521, 31–39. [Google Scholar] [CrossRef]
- Arab, N.; Fotouhi, L.; Salis, A.; Dorraji, P. An amplified electrochemical sensor employing a polymeric film and graphene quantum dots/multiwall carbon nanotubes in a deep eutectic solvent for sensitive analysis of paracetamol and 4-aminophenol. New J. Chem. 2020, 44, 15742–15751. [Google Scholar] [CrossRef]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent trends in macro-,micro-, and nanomaterial-based tools and strategies for heavymetal detection. Chem Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, I.; Eremia, S.; Kusko, M.; Radoi, A.; Vasile, E.; Radu, G. Molybdenum disulphide and graphene quantum dots as electrode modifiers for laccase biosensor. Biosens. Bioelectron. 2016, 75, 232–237. [Google Scholar] [CrossRef] [PubMed]



| Source | Method | Application | Ref. |
|---|---|---|---|
| wood charcoal | Electrochemical oxidation | Detection of H2O2 and glucose | [27] |
| coal tar pitch | Chemical oxidation | Fluorescent probes | [35] |
| graphene oxide | Chemical oxidation | Fluorescent nano-probes | [36] |
| graphene oxide | HTC | Fluorescent probes for bio-imaging | [38] |
| coke | Electrochemical oxidation | Fluorescent properties for multicolor light-emitting diode devices | [40] |
| citric acid and sodium citrate | Electrochemical oxidation | Tests of the mutagenicity | [41] |
| graphite plate | Laser ablation | Fluorescent probes | [42] |
| graphite flakes | Laser ablation | Production of sulfur-doped graphene nanosheets | [43] |
| glucose | Controllable synthesis and HTC | Electrochemical luminescence devices | [46] |
| citric acid | Controllable synthesis | Determination of GQDs properties | [47] |
| honey | Pyrolysis | Biocompatible fluorescent ink | [31] |
| glucose | Pyrolysis | Chemiluminescent biosensor for the detection of cholesterol | [48] |
| Bougainvillea spectabilis flowers | Carbonization | Electrodes for detention of catechin | [49] |
| rice grains | Pyrolysis | Fluorescent properties | [50] |
| rice husk | Carbonization and HTC | Test for biocompatibility | [28] |
| coffee grounds | HTC | Bio-imaging | [9] |
| durian | HTC | Bio-imaging | [52] |
| bamboo wood | HTC | fluorescence sensors | [53] |
| corn powder | HTC | Solar cells | [55] |
| glucose powder | HTC | Determination of energy levels | [57] |
| cellulose | HTC | Cell imaging | [58] |
| citric acid | HTC | Detection of doxorubicin | [62] |
| sucrose | HTC | Photocatalytic activity | [56] |
| citric acid and ethylenediamine | HTC | QY of GQDs | [60] |
| citric acid | HTC | Fluorescent biosensor for TYR and ACP | [63] |
| citric acid | HTC | Fluorescent sensor of Hg2+ | [61] |
| citric acid and lignin-sulfonate | HTC | Fluorescent sensors for Fe3+ | [64] |
| tea waste | Microwave | Sensors for the detection of the Fe3+ | [66] |
| mango leaves | Microwave | Detection of intracellular temperature | [32] |
| cow’s milk | Microwave | Drug delivery | [69] |
| citric acid and thiourea | Microwave | Fluorescent probes for bio-imaging | [70] |
| grape seed extract | Microwave | Photoluminescent Sensing Applications | [71] |
| fructose | Microplasma | Sensors for silver ions | [68] |
| GQDs Based Sensor | Detection Method | Analytes | Linear Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|---|---|
| GQD-RuCl3/CCE | DPV | L-Tyr | 1–937 | 0.23 | [92] |
| β-CD-GQD/GCE | DPV | L-T | 0.1–1.5 | 0.1 | [93] |
| (GQDs-NHCH2CH2NH)/GCE | DPV | DA | 1–150 | 0.115 | [94] |
| CS/N,GQDs@SPCE | DPV | DA | 1–200 | 0.145 | [95] |
| GQD-CS-CPE | SWV AMP | EP | 0.36–380 | 0.0003 | [96] |
| His-GQD-GMA | DPV | DA | 0.001–80 | 0.29 nM | [97] |
| GQDs/GCE | DPV | DA | 0.4–100 | 0.05 | [98] |
| GQDs@MWCNTs/GCE | CV | DA | 0.25–250 | 0.095 | [99] |
| GQD-TMSPED-AuNC | AMP | DA EP | 0.005–2.1 0.01–4 | 0.005 0.01 | [100] |
| GCE/GQDs/Lac | CV | EP | 1–120 | 0.083 | [101] |
| GCE/GQDs/AuNPs | SWSV | NEP | 0.5–7.5 | 0.15 | [102] |
| GQDs-PSSA/GO/GCE | DPV | E P | 0.001–6 0.001–6 | 0.23 0.31 | [103] |
| Fe3O4@GQD/fMWCNTs/GCE | DPV | P | 0.0031–0.945 | 0.00063 | [104] |
| NGQD@NC@Pd/GCE | AMP | H2O2 | 1400 | 0.02 | [105] |
| Chit-GQDs/AgNCs/GE | AMP | H2O2 | 10–7380 | 0.15 | [106] |
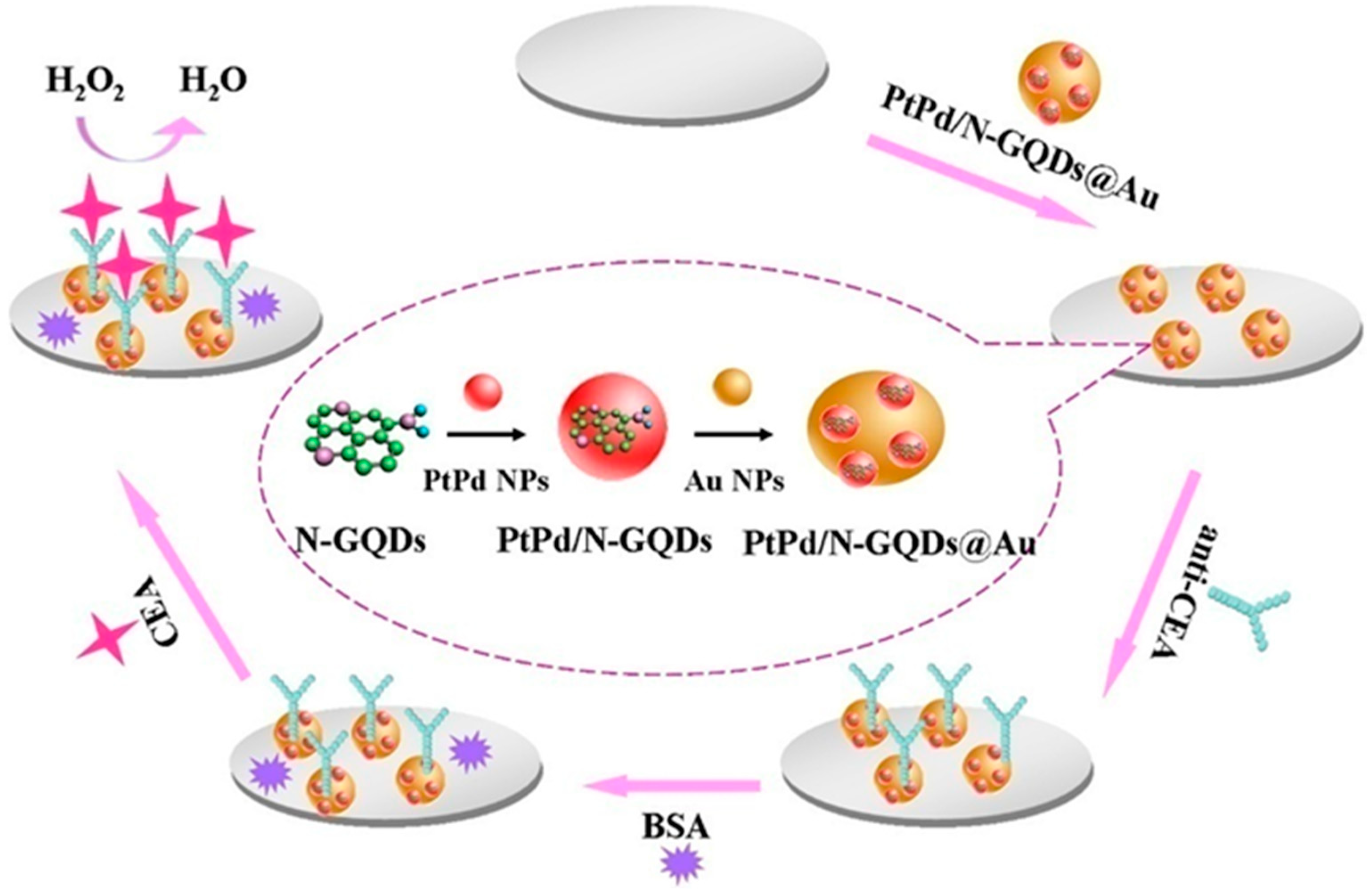
| PtPd/N-GQDs@Au | AMP | CEA-H2O2 | 5 × 10−5–0.05 | 2 × 10−9 | [107] |
| β-CD-GQDs/GCE | SWV | AC | 0.01–170 | 0.49 | [108] |
| GQD/AuNP/GCE | DPV | Q | 0.01–6.0 | 0.002 | [109] |
| DNA/GQDs/GCE | CV, DPV | DNA | 0.01–0.5 | 0.001 | [110] |
| PDDA-GO/GQDs/DNA-gold NPs | ECL | DNA | 1.0 × 10−6–1.0 | 1.0 × 10−7 | [111] |
| GQDs@ La3 + @ZrO2/GCE | CV; EIS | FL | 0.00175–15.75 | 0.00082 | [112] |
| (mag@MIP)-GQDs-FG-NF/SPE | SWV | EE | 10 × 10−3–2.5 | 0.0026 | [113] |
| GQDs/GCE | DPV | HY CA | 4.0–600 6–400 | 0.40 0.75 | [114] |
| GQDs/graphene/GCE | DPASV | Cu2+ | 0.015–8.775 | 0.00134 | [115] |
| GCE/PTH-afGQDs | CV | Hg2+ | 1 × 10−6–1 | 6 × 10−7 | [116] |
| N-doped GQD/ITO | CV,EIS- | Hg2+ | 0.05–0.25 | 10 ppb | [117] |
| PANI/GQD-modified SPCE | LSV | Cr6+ | 100–1000 | 97 | [118] |
| luminol-NGQDs | ECL | H2O2 | 0.033~74 | 0.01 | [119] |
| (Fe3O4@GODs/f-MWCNTs/GCE | DPV | L-DOPA | 3–400 | 14.3 | [120] |
| PPy/GQDs@PB/GF | CVs-CA | L-cys | 0.2–50 50–1000 | 0.15 | [121] |
| NMO/GQDs/CS/GCEox | DPV | DZ | 0.1–330 | 0.027 | [122] |
| GQDs-α-CD-AgNPs-GCE | CV | AFM1 | 15–25,000 | 2 | [123] |
| BSA/anti-OTA/GQDs@ZrO2/ITO | CV,DPV | OTA | 1–20 ng/mL | 0.38 ng/mL | [124] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bressi, V.; Ferlazzo, A.; Iannazzo, D.; Espro, C. Graphene Quantum Dots by Eco-Friendly Green Synthesis for Electrochemical Sensing: Recent Advances and Future Perspectives. Nanomaterials 2021, 11, 1120. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051120
Bressi V, Ferlazzo A, Iannazzo D, Espro C. Graphene Quantum Dots by Eco-Friendly Green Synthesis for Electrochemical Sensing: Recent Advances and Future Perspectives. Nanomaterials. 2021; 11(5):1120. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051120
Chicago/Turabian StyleBressi, Viviana, Angelo Ferlazzo, Daniela Iannazzo, and Claudia Espro. 2021. "Graphene Quantum Dots by Eco-Friendly Green Synthesis for Electrochemical Sensing: Recent Advances and Future Perspectives" Nanomaterials 11, no. 5: 1120. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051120






