Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Procedures
2.2.1. Preparation of Silver–Iron Oxide Nanoparticles
- –
- One part was solubilized with 10% hydrochloric acid solution (gravimetrically);
- –
- The second part was solubilized with sodium thiosulfate 10% solution (gravimetrically);
- –
- The third part was solubilized with ammonia 10% solution (gravimetrically).
2.2.2. Obtaining Liquid Membranes on Polypropylene Support
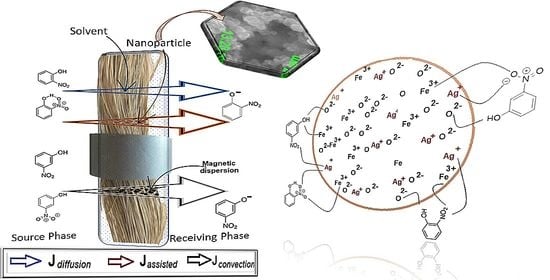
2.2.3. Pertraction of o- and m-Nitrophenols with Impregnated Liquid Membranes
2.3. Equipment
3. Results
4. Discussion
4.1. Obtaining and Characterizing the Nanoparticles
4.2. Process Performance of the Membrane System with Silver–Iron Oxide Magnetic Nanoparticles in n-Alcohols on Polypropylene Fiber Support
4.2.1. The Effect of the Nanoparticle Composition
4.2.2. The Effect of the Oscillating Magnetic Field
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Bruggen, B. Opening Ceremony; European Membrane Society Summer School: Bucharest, Romania, 14–19 June 2010; Unpublished Presentation.
- Liao, Z.; Zhu, J.; Li, X.; Van der Bruggen, B. Regulating composition and structure of nanofillers in thin film nanocomposite (TFN) membranes for enhanced separation performance: A critical review. Sep. Purif. Technol. 2021, 266, 118567. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Tan, Y.H.; Chong, C.Y.; Krause-Rehberg, R.; Awad, S. Tailor-made thin film nanocomposite membrane incorporated with graphene oxide using novel interfacial polymerization technique for enhanced water separation. Chem. Eng. J. 2018, 344, 524–534. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Shamsabadi, A.A.; Aktij, S.A.; Seyedfour, S.F.; Sharifian Gh, M.; Rahimpour, A.; Esfahani, M.R.; Ulbricht, M.; Soroush, M. Exploiting synergetic effects of graphene oxide and a silver-based metal-organic; framework to enhance antifouling and anti-biofouling properties of thin-film nanocomposite membranes. ACS Appl. Mater. Interfaces 2018, 10, 42967–42978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Y.; Pan, G.; Xu, J.; Yan, H.; Liu, Y. In situ formation of copper nanoparticles in carboxylated chitosan layer: Preparation and characterization of surface modified TFC membrane with protein fouling resistance and long-lasting antibacterial properties. Sep. Purif. Technol. 2017, 176, 164–172. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Pereira, J.M.; Trilles, C.A.; Aquino, R.R.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Performance and antifouling behavior of thin-film nanocomposite nanofiltration membranes with embedded silica spheres. Sep. Purif. Technol. 2019, 210, 521–529. [Google Scholar] [CrossRef]
- Abdelsamad, A.M.A.; Khalil, A.S.G.; Ulbricht, M. Influence of controlled functionalization of mesoporous silica nanoparticles as tailored fillers for thin-film nanocomposite membranes on desalination performance. J. Membr. Sci. 2018, 563, 149–161. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Kumar, M.; Chetty, R. Morphological effect of ZnO nanostructures on desalination performance and antibacterial activity of thin-film nanocomposite (TFN) membrane. Desalination 2020, 495, 114673. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Zhu, J.; Zhang, Y.; Liu, J.; van der Bruggen, B. Construction of TiO2@graphene oxide incorporated antifouling nanofiltration membrane with elevated filtration performance. J. Membr. Sci. 2017, 533, 279–288. [Google Scholar] [CrossRef]
- Hradil, J.; Krystl, V.; Hrabanek, P.; Bernauer, B.; Kocirık, M. Heterogeneous membranes based on polymeric adsorbents for separation of small molecules. React. Funct. Polym. 2004, 61, 303–313. [Google Scholar] [CrossRef]
- Liu, C.; Faria, A.F.; Ma, J.; Elimelech, M. Mitigation of biofilm development on thin film composite membranes functionalized with zwitterionic polymers and silver nanoparticles. Environ. Sci. Technol. 2017, 51, 182–191. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Taheri-Kafrani, A.; Asadnia, M.; Razmjou, A. Bienzymatic modification of polymeric membranes to mitigate biofouling. Sep. Purif. Technol. 2020, 237, 116464. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Hu, Y.; Sun, S. Membrane technology in wastewater treatment enhanced by functional nanomaterials. J. Clean. Prod. 2018, 197, 339–348. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Woo, S.T.; Yun, T.; Kwak, S.-Y. Fouling-resistant microfiltration membrane modified with magnetite nanoparticles by reversible conjunction. Sep. Purif. Technol. 2018, 202, 299–306. [Google Scholar] [CrossRef]
- Ghaemi, N.; Madaeni, S.S.; Daraei, P.; Rajabi, H.; Zinadini, S.; Alizadeh, A.; Heydari, R.; Beygzadeh, M.; Ghouzivand, S. Polyethersulfone membrane enhanced with iron oxide nanoparticles for copper removal from water: Application of new functionalized Fe3O4 nanoparticles. Chem. Eng. J. 2015, 263, 101–112. [Google Scholar] [CrossRef]
- Agbaje, T.A.; Al-Gharabli, S.; Mavukkandy, M.O.; Kujawa, J.; Arafat, H.A. PVDF/magnetite blend membranes for enhanced flux and salt rejection in membrane distillation. Desalination 2018, 436, 69–80. [Google Scholar] [CrossRef]
- Massart, R.; Dubois, E.; Cabuil, V. Preparation and Properties of Monodisperse Magnetic Fluids. J. Magn. Magn. Mater. 1995, 149, 1–5. [Google Scholar] [CrossRef]
- Massart, R.; Roger, J.; Cabuil, V. New Trends in chemistry of Magnetic colloids: Polar and Nonpolar Magnetic Fluids, Emulsions, Capsules and Vesicles. Braz. J. Phys. 1995, 25, 135–141. [Google Scholar]
- Zhang, L.; He, R.; Gu, H.C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Yan, A.G.; Liu, X.; Qiu, G.; Wu, H.; Yi, R.; Zhang, N.; Xu, J. Solvothermal synthesis and characterization of size-controlled Fe3O4 nanoparticles. J. Alloys Compd. 2008, 458, 487–491. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Meng, C.G. Facile fabrication of Fe3O4 and Co3O4 microspheres and their influence on the thermal decomposition of ammonium perchlorate. J. Alloys Compd. 2016, 674, 259–265. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H.; Amiri, S. Ferrite-based magnetic nanofluids used in hyperthermia applications. J. Magn. Magn. Mater. 2012, 324, 903–915. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep. Purif. Technol. 2009, 68, 312–319. [Google Scholar] [CrossRef]
- Scherer, C.; Figueiredo Neto, A.M. Ferrofluids: Properties and Applications. Braz. J. Phys. 2005, 35, 718–727. [Google Scholar] [CrossRef]
- Marinescu, G.; Patron, L.; Culita, C.D.; Neagoe, C.; Lepadatu, I.C.; Balint, I.; Bessais, L.; Cizmas, C.B. Synthesis of magnetite nanoparticles in the presence of aminoacids. J. Nanopart. Res. 2006, 8, 1045–1051. [Google Scholar] [CrossRef]
- Vidal-Vidal, J.; Rivas, J.; López-Quintela, M.A. Synthesis of monodisperse maghemite nanoparticles by the microemulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 44–51. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.J.; Sun, S.H. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef]
- Shokrollahi, H. Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids. Mater. Sci. Eng. C 2013, 33, 2476–2487. [Google Scholar] [CrossRef]
- Setyawan, H.; Widiyastuti, W. Progress in the Preparation of Magnetite Nanoparticles through the Electrochemical Method. KONA Powder Part. J. 2019, 36, 145–155. [Google Scholar] [CrossRef]
- Fajaroh, F.; Setyawan, H.; Widiyastuti, W.; Winardi, S. Synthesis of magnetite nanoparticles by surfactant-free electrochemical method in an aqueous system. Adv. Powder Technol. 2012, 23, 328–333. [Google Scholar] [CrossRef]
- Nurlilasari, P.; Widiyastuti, W.; Setyawan, H.; Faizal, F.; Wada, M.; Lenggoro, I.W. High-throughput production of magnetite nanoparticles prepared by the monopolar arrangement of iron electrodes in water. Chem. Eng. Sci. 2019, 201, 112–120. [Google Scholar] [CrossRef]
- Franger, S.; Berthet, P.; Berthon, J. Electrochemical synthesis of Fe3O4 nanoparticles in alkaline aqueous solutions containing complexing agents. J. Solid State Electrochem. 2004, 8, 218–223. [Google Scholar] [CrossRef]
- Gopi, D.; Thameem Ansari, M.; Kavitha, L. Electrochemical synthesis and characterization of cubic magnetite nanoparticle in aqueous ferrous perchlorate medium. Arab. J. Chem. 2016, 9, S829–S834. [Google Scholar] [CrossRef] [Green Version]
- Sawada, I.; Fachrul, R.; Ito, T.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties. J. Membr. Sci. 2012, 387–388, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mollahosseini, A.; Rahimpour, A.; Jahamshahi, M.; Peyravi, M.; Khavarpour, M. The effect of silver nanoparticle size on performance and antibacteriality of polysulfone ultrafiltration membrane. Desalination 2012, 306, 41–50. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Pedro Alvarez, J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sileikaite, A.; Prosycevas, I.; Puiso, J.; Juraitis, A.; Guobien, A. Analysis of Silver Nanoparticles Produced by Chemical Reduction of Silver Salt Solution. Mater. Sci. 2006, 12, 287–291. [Google Scholar]
- Pulit-Prociak, J.; Banach, M. Silver nanoparticles—A material of the future…? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Okafor, F.; Janen, A.; Kukhtareva, T.; Edwards, V.; Curley, M. Green synthesis of silver nanoparticles, their characterization, application, and antibacterial activity. Int. J. Environ. Res. Publth Health 2013, 10, 5221–5238. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Upadhyaya, L.; Semsarilar, M.; Deratani, A.; Quemener, D. Nanocomposite membranes with magnesium, titanium, iron and silver nanoparticles-A review. J. Membr. Sci. Res. 2017, 3, 187–198. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Ren, B.; Huang, Y.; Wang, D.; Liao, Z.; Li, Q.; Wang, L.; Dionysiou, D.D. Polymeric ultrafiltration membrane with in situ formed nano-silver within the inner pores for simultaneous separation and catalysis. J. Membr. Sci. 2019, 579, 190–198. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Arul, H.; Bosak, V.Z.; Burban, A.F.; Voice, T.C.; Bruening, M.L.; Tarabara, V.V. Effect of filler incorporation route on the properties of polysulfone–silver nanocomposite membranes of different porosities. J. Membr. Sci. 2008, 325, 58–68. [Google Scholar] [CrossRef]
- Bartsch, R.A.; Way, J.D. (Eds.) Chemical Separations with Liquid Membranes; ACS: Washington, DC, USA, 1996; pp. 208–221. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, T.; Nishiki, T.; Osaki, K.; Muto, A. A practicable process for phenol removal with liquid surfactant membrane permeation column. Sep. Sci. Technol. 1997, 32, 1447–1462. [Google Scholar] [CrossRef]
- Garea, A.; Urtiaga, A.M.; Ortiz, M.I.; Alonso, A.I.; Irabiem, J.A. Phenol recovery with SLM using cyanex 923. Chem. Eng. Commun. 1993, 120, 85–97. [Google Scholar] [CrossRef]
- Cahn, R.P.; Li, N.N. Separation of phenol from wastewater by the liquid membrane technique. Sep. Sci. Technol. 1974, 9, 505–519. [Google Scholar] [CrossRef]
- Chang, Y.C.; Li, S.P. A study of emulsified liquid membrane treatment of phenolic wastewater. Desalination 1983, 47, 351–361. [Google Scholar] [CrossRef]
- Boyadzhiev, L.; Benenshek, E.; Lazarova, Z. Removal of phenol from wastewater by double emulsion membranes and creeping film pertraction. J. Membr. Sci. 1984, 21, 137–144. [Google Scholar] [CrossRef]
- Gadekar, P.T.; Mukkolath, A.V.; Tiwari, K.K. Recovery of nitrophenols from aqueous solutions by a liquid emulsion membrane system. Sep. Sci. Technol. 1992, 27, 427–445. [Google Scholar] [CrossRef]
- Kakoi, T.; Goto, M.; Natsukawa, S.; Lkemizu, K.; Nakashio, F. Recovery of phenols using liquid surfactant membranes prepared with newly synthesized surfactants. Sep. Sci. Technol. 1996, 31, 107–124. [Google Scholar] [CrossRef]
- Ghimpusan, M.; Nechifor, G.; Din, I.S.; Nechifor, A.C.; Passeri, P. Application of Hollow Fibre Membrane Bioreactor Instead of Granular Activated Carbon Filtration for Treatment of Wastewater from Car Dismantler Activity. Mater. Plast. 2016, 53, 578–584. [Google Scholar]
- Din, I.S.; Cimbru, A.M.; Rikabi, A.A.K.K.; Tanczos, S.K.; Ticu (Cotorcea), S.; Nechifor, G. Iono-molecular Separation with Composite Membranes VI. Nitro-phenol separation through sulfonated polyether ether ketone on capillary polypropylene membranes. Rev. Chim. 2018, 69, 1603–1607. [Google Scholar] [CrossRef]
- Nechifor, A.C.; Cotorocea, S.; Bungău, C.; Albu, P.C.; Oprea, O.; Grosu, A.R.; Pîrţac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles Cellulose Derivates for Air Odor Correction. Membranes 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, I.; Gîrdea, R.; Cristea, C.; Nechifor, G.; Ruse, E.; Totu, E.E. Removal and recovery of some phenolic pollutants using liquid membranes. Rom. Biotechnol. Lett. 2010, 15, 5702–5708. [Google Scholar]
- Koter, S.; Szczepański, P.; Mateescu, M.; Nechifor, G.; Badalau, L.; Koter, I. Modeling of the cadmium transport through a bulk liquid membrane. Sep. Purif. Technol. 2013, 107, 135–143. [Google Scholar] [CrossRef]
- Diaconu, I.; Aboul-Enein, H.Y.; Al-Omar, M.A.; Nechifor, G.; Ruse, E.; Bunaciu, A.A.; Totu, E.E. Separation of nitrophenols. Equilibriums in bi-and tri-phasic systems. Arab. J. Chem. 2011, 4, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Diaconu, I.; Nechifor, G.; Nechifor, A.C.; Totu, E.E.; Ruse, E. The transport of nitrophenols through liquid membranes. Rev. Chim. 2009, 60, 1243–1246. [Google Scholar]
- Diaconu, I.; Nechifor, G.; Nechifor, A.C.; Ruse, E.; Totu, E.E. Membrane techniques used at the separation of some phenolic compounds from aqueous media. Sci. Bull. B Chem. Mater. Sci. UPB 2009, 71, 39–46. [Google Scholar]
- Nafliu, I.M.; Al-Ani, H.N.A.; Grosu (Miron), A.R.; Albu, P.C.; Nechifor, G. Iono-molecular separation with composite membranes. viii. recuperative aluminium ions separation on capilary polypropylene S-EPDM composite membranes. Mater. Plast. 2019, 56, 32–36. [Google Scholar] [CrossRef]
- Szczepański, P.; Diaconu, I. Transport of p-nitrophenol through an agitated bulk liquid membrane. Sep. Sci. Technol. 2012, 47, 1725–1732. [Google Scholar] [CrossRef]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron (II) with 1,10-phenanthroline in the presence of large amounts of iron (III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef]
- El-Ghamry, M.T.; Frei, R.W. Spectrophotometric determination of trace amounts of silver( I). Anal. Chem. 1968, 40, 1986–1990. [Google Scholar] [CrossRef]
- Lee, K.; Clydesdale, F.M. Quantitative determination of the elemental, ferrous, ferric, soluble, and complexed iron in foods. J. Food Sci. 1979, 44, 549–554. [Google Scholar] [CrossRef]
- Omidi, F.; Behbahani, M.; Shahtaheri, S.J.; Salimi, S. Trace monitoring of silver ions in food and water samples by flame atomic absorption spectrophotometry after preconcentration with solvent-assisted dispersive solid phase extraction. Environ. Monit. Assess. 2015, 187, 361. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, L.; Semsarilar, M.; Quemener, D.; Fernández-Pacheco, R.; Martinez, G.; Coelhoso, I.M.; Nunes, S.P.; Crespo, J.G.; Mallada, R.; Portugal, C.A.M. Block copolymer-based magnetic mixed matrix membranes—Effect of magnetic field on protein permeation and membrane fouling. Membranes 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]












| Material | Polypropylene (PP) |
|---|---|
| Porosity | 40–50% |
| Dimension of pore | 0.002–0.2 µm |
| External diameter | 0.45 mm |
| pH | 1–14 |
| T (°C) | Tmax = 50 °C |
| Fascicle dimensions | Φ 25 × 750 mm2 |
| Filtration surface (fascicle) | 1.0 m² |
| Operation pressure | 0.1–0.4 bar |
| The average flow of permeate | 10–15 L/m²h |
| Liquid Membrane with Nanoparticles | NP1 | NP2 | NP3 | ||||
|---|---|---|---|---|---|---|---|
| C10–OH | C8–OH | C10–OH | C8–OH | C10–OH | C8–OH | ||
| J, (g h−1 m−2) | o-Nitrophenol | 194 + 2 | 186 + 2 | 187 + 2 | 172 + 2 | 198 + 2 | 192 + 2 |
| m-Nitrophenol | 188 + 2 | 182 + 2 | 180 + 2 | 168 + 2 | 191 + 2 | 186 + 2 | |
| EE (%) | o-Nitrophenol | 97 + 1 | 93 + 1 | 93.5 + 1 | 86 + 1 | 99 + 1 | 96 + 1 |
| m-Nitrophenol | 94 + 1 | 91 + 1 | 99 + 1 | 84 + 1 | 95.5 + 1 | 93 + 1 | |
| No. | Silver Source | Composition (%) | |||||
|---|---|---|---|---|---|---|---|
| Ag | Cl | Br | I | S | Other | ||
| 1. | Titration of chloride ions | 75.26 | 24.73 | - | - | - | - |
| 2. | Titration of chloride and iodide ions (separately and in a mixture) | 74.38 | 20.10 | - | 5.51 | - | - |
| 3. | Photo labs (classical processed) | 67.43 | 10.32 | 12.67 | - | 9.17 | Organic impurities |
| 4. | Determination of drinking water characteristics; general cations separation; research activities. | 65.88 | 20.03 | 9.24 | 4.54 | - | Inorganic impurities (metallic ions) |
| The Specific Spectral Line Used | Nanoparticles | |||||
|---|---|---|---|---|---|---|
| NP1 | NP2 | NP3 | ||||
| Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | |
| O K | 41.52 | 71.41 | 29.2 | 59.05 | 38.02 | 65.39 |
| Ag L | 0.93 | 0.24 | 0.21 | 0.06 | 1.12 | 0.28 |
| Fe K | 57.55 | 28.35 | 70.59 | 40.89 | 60.86 | 34.33 |
| The Specific Metal | Nanoparticles | |||||
|---|---|---|---|---|---|---|
| NP1 | NP2 | NP3 | ||||
| UV-Vis | AAS | UV-Vis | AAS | UV-Vis | AAS | |
| Ag (%) | 1.04 + 0.04 | 1.038 + 0.003 | 0.23 + 0.07 | 0.228 + 0.005 | 1.20 + 0.03 | 1.219 + 0.001 |
| Fe (%) | 56.88 + 0.07 | 56.854 + 0.005 | 69.98 + 0.08 | 70.146 + 0.009 | 59.87 + 0.04 | 59.900 + 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimulescu, I.A.; Nechifor, A.C.; Bǎrdacǎ, C.; Oprea, O.; Paşcu, D.; Totu, E.E.; Albu, P.C.; Nechifor, G.; Bungău, S.G. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials 2021, 11, 1204. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051204
Dimulescu IA, Nechifor AC, Bǎrdacǎ C, Oprea O, Paşcu D, Totu EE, Albu PC, Nechifor G, Bungău SG. Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes. Nanomaterials. 2021; 11(5):1204. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051204
Chicago/Turabian StyleDimulescu (Nica), Ioana Alina, Aurelia Cristina Nechifor, Cristina Bǎrdacǎ (Urducea), Ovidiu Oprea, Dumitru Paşcu, Eugenia Eftimie Totu, Paul Constantin Albu, Gheorghe Nechifor, and Simona Gabriela Bungău. 2021. "Accessible Silver-Iron Oxide Nanoparticles as a Nanomaterial for Supported Liquid Membranes" Nanomaterials 11, no. 5: 1204. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051204




.jpg)