First-Principles Insight into Pd-Doped C3N Monolayer as a Promising Scavenger for NO, NO2 and SO2
Abstract
:1. Introduction
2. Computation Methods
3. Results and Discussions
3.1. Isolated Gas Molecules and Pd-C3N Monolayer
3.2. Adsorption Analysis of Pd-C3N Monolayer to NO, NO2, SO2
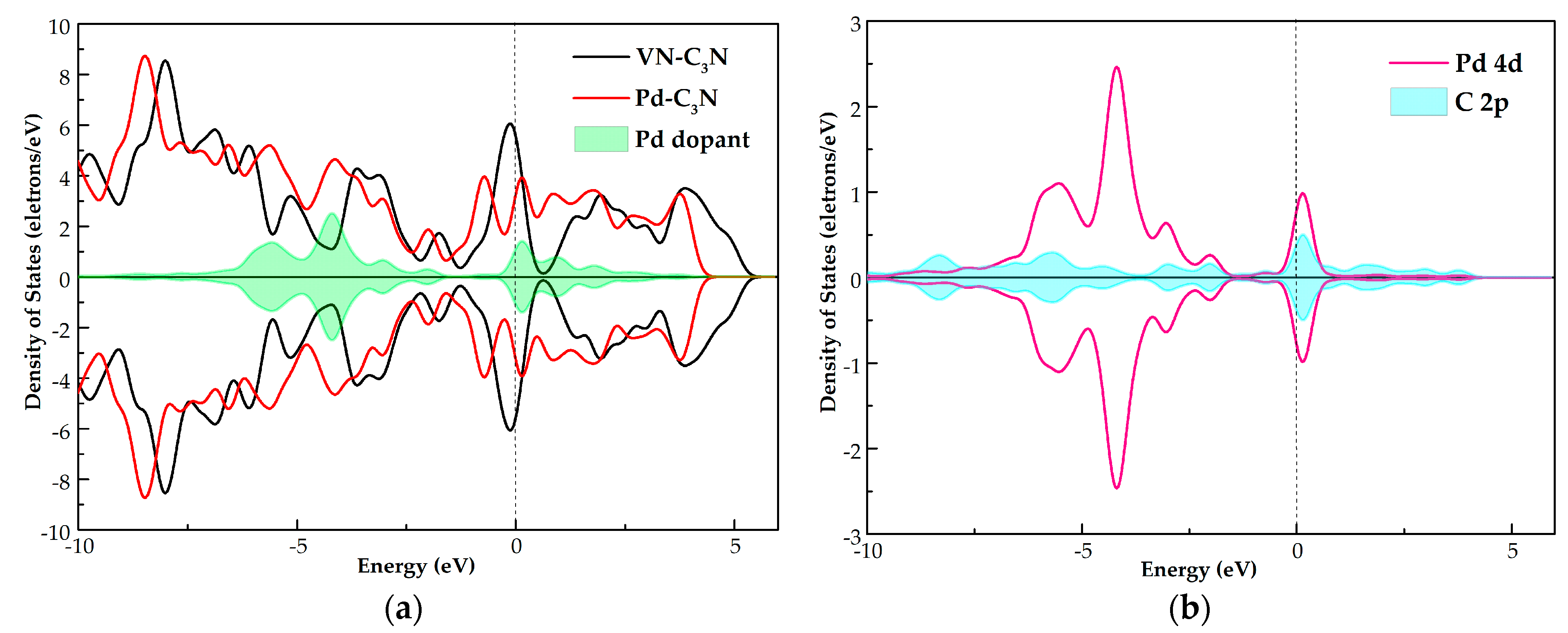
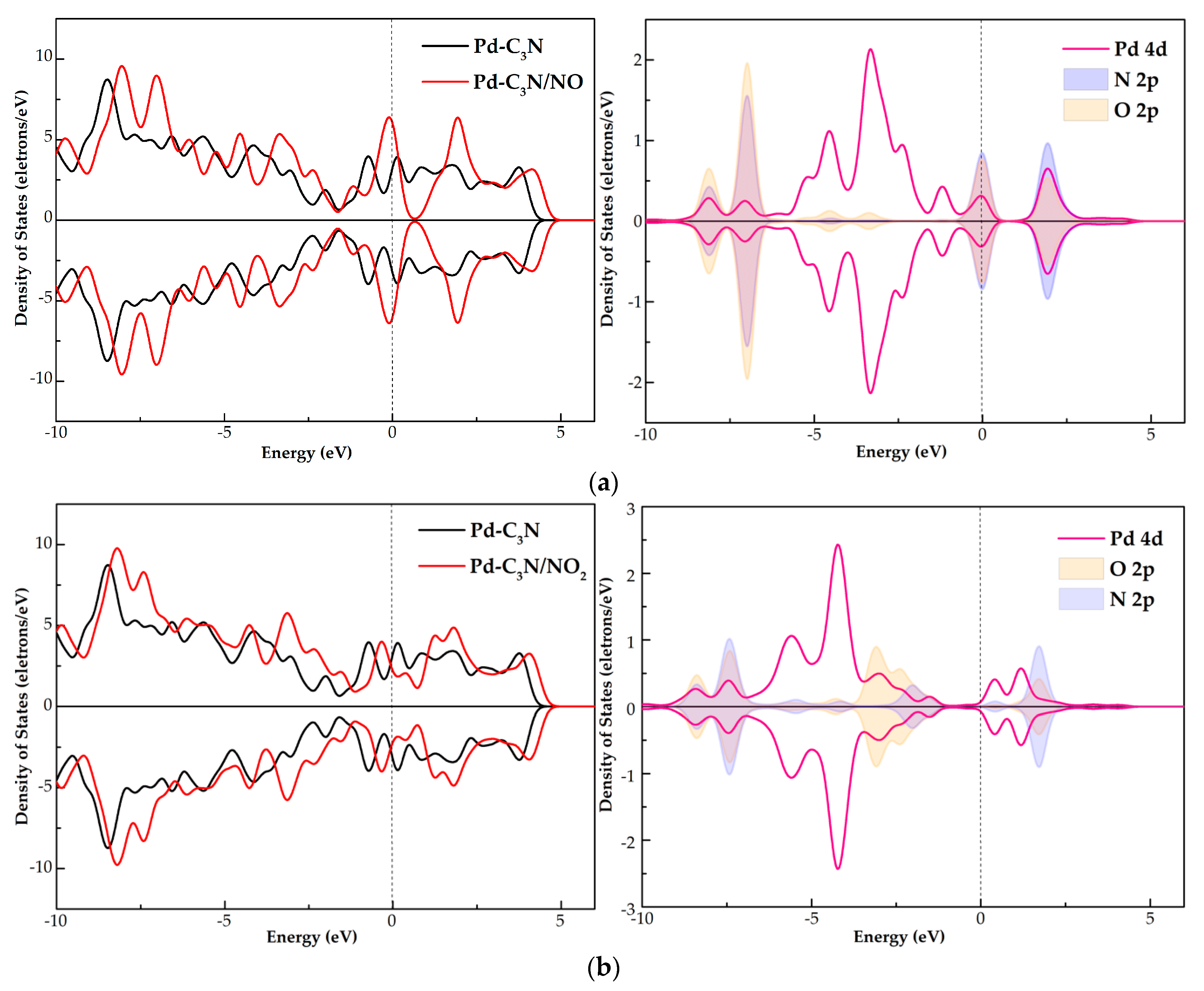
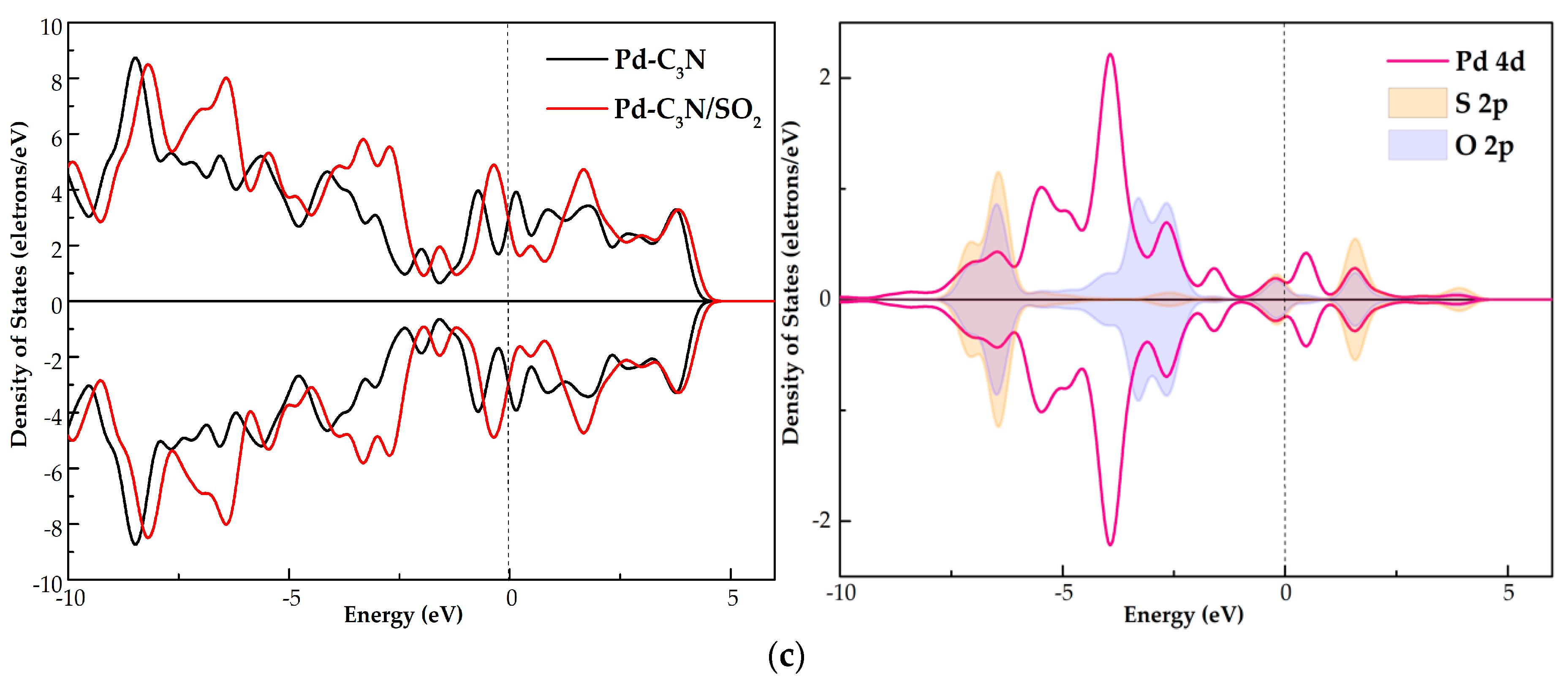
3.3. DOS Analysis of NO, NO2 and SO2 Adsorption Systems
3.4. Band Structure Analysis of NO, NO2 and SO2 Systems
4. Conclusions
- The Pd dopant is more likely to be adsorbed on the N-vacancy site of the C3N than the C-vacancy site, because the lower binding energy (Eb = −5.023 eV) of this doping system implies a more stable structure.
- NO, NO2 and SO2 can be stably adsorbed by the Pd-C3N monolayer and the adsorption can be identified as chemisorption. Besides, the adsorption energy (Ead) of the Pd-C3N/gas system is much higher than that of the C3N/gas system. Among three gas adsorption systems, Ead and QT of the NO2 system are the largest, which indicates that Pd-C3N has the strongest adsorption performance for NO2.
- Through the analysis of DOS, it is found that the gas molecules are activated during the interaction with the Pd dopant surface. The orbital hybridization of these activated states with Pd 4d give rise to new peaks in the TDOS of the three adsorption systems, which influences the electronic behavior of Pd-C3N.
- Through analyzing the band structure, it can be discovered that the band gap of Pd-C3N becomes narrower after adsorbing NO, NO2 and SO2, which significantly improves the conductivity of Pd-C3N, especially after adsorbing NO and SO2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Louw, I. Potential radiological impact of the phosphate industry in South Africa on the public and the environment (Paper 1). J. Environ. Radioact. 2020, 217, 106214. [Google Scholar] [CrossRef] [PubMed]
- Rybicka, E.H. Impact of mining and metallurgical industries on the environment in Poland. Appl. Geochem. 1996, 11, 3–9. [Google Scholar] [CrossRef]
- Li, B.L.; Zhou, Q.; Peng, R.C.; Liao, Y.M.; Zeng, W. Adsorption of SF6 decomposition gases (H2S, SO2, SOF2 and SO2F2) on Sc-doped MoS2 surface: A DFT study. Appl. Surf. Sci. 2021, 549, 149271. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, T.R.; Zhou, B.; Cui, Y.T.; Yan, H.; Liu, Z.K.; Schmitt, F.; Lee, J.; Moore, R.; Chen, Y.L.; et al. Direct observation of the transition from indirect to direct bandgap in atomically thin epitaxial MoSe2. Nat. Nanotechnol. 2014, 9, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zeng, Z.Y.; Zhang, H. Metal dichalcogenide nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2013, 42, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Xia, N.; Wu, X.J.; Li, Z.Y.; Yang, J.L. Silicene as a highly sensitive molecule sensor for NH3, NO and NO2. Phys. Chem. Chem. Phys. 2014, 16, 6957–6962. [Google Scholar] [CrossRef]
- Hu, W.; Wu, X.J.; Li, Z.Y.; Yang, J.L. Helium separation via porous silicene based ultimate membrane. Nanoscale 2013, 5, 9062–9066. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.Q.; Hu, W.; Li, Z.Y.; Yang, J.L. A first-principles study of gas adsorption on germanene. Phys. Chem. Chem. Phys. 2014, 16, 22495–22498. [Google Scholar] [CrossRef]
- Kaloni, T.P. Tuning the structural, electronic, and magnetic properties of germanene by the adsorption of 3d transition metal atoms. J. Phys. Chem. C 2014, 118, 25200–25208. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.Q.; Tao, C.; Bai, Y.; Liu, S.P. Adsorption of nitrogen based gas molecules on noble metal functionalized carbon nitride nanosheets: A theoretical investigation. Comput. Theor. Chem. 2021, 1194, 112950. [Google Scholar] [CrossRef]
- Nagarajan, V.; Chandiramouli, R. Adsorption behavior of NH3 and NO2 molecules on stanene and stanane nanosheets—A density functional theory study. Chem. Phys. Lett. 2018, 695, 162–169. [Google Scholar] [CrossRef]
- Pierucci, D.; Zribi, J.; Henck, H.; Chaste, J.; Silly, M.G.; Bertran, F.; Le Fèvre, P.; Gil, B.; Summerfield, A.; Beton, P.H.; et al. Van der Waals epitaxy of two-dimensional single-layer h-BN on graphite by molecular beam epitaxy: Electronic properties and band structure. Appl. Phys. Lett. 2018, 112, 253102. [Google Scholar] [CrossRef]
- Zhou, Q.; Zeng, W.; Chen, W.G.; Xu, L.N.; Kumar, R.; Umar, A. High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Actuators B Chem. 2019, 298, 126870. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009, 54, 1–67. [Google Scholar] [CrossRef]
- Zhou, Q.; Umar, A.; Sodki, E.; Amine, A.; Xu, L.N.; Gui, Y.G.; Ibrahim, A.A.; Kumar, R.; Baskoutas, S. Fabrication and characterization of highly sensitive and selective sensors based on porous NiO nanodisks. Sens. Actuators B Chem. 2018, 259, 604–615. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in grapheme. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Sprinkle, M.; Siegel, D.; Hu, Y.; Hicks, J.; Tejeda, A.; Taleb-Ibrahimi, A.; Le Fevre, P.; Bertran, F.; Vizzini, S.; Enrique, H.; et al. First direct observation of a nearly ideal graphene band structure. Phys. Rev. Lett. 2009, 103, 226803. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, X.X.; Li, Y.; Chen, D.C.; Zhang, Y. First-principles insight into Ni-doped InN monolayer as a noxious gases scavenger. Appl. Surf. Sci. 2019, 494, 859–866. [Google Scholar] [CrossRef]
- Cui, H.; Liu, T.; Zhang, Y.; Zhang, X.X. Ru-InN monolayer as a gas scavenger to guard the operation status of SF6 insulation devices: A first-principles theory. IEEE Sens. J. 2019, 19, 5249–5255. [Google Scholar] [CrossRef]
- Sajjad, M.; Feng, P. Study the gas sensing properties of boron nitride nanosheets. Mater. Res. Bull. 2014, 49, 35–38. [Google Scholar] [CrossRef]
- Ma, D.W.; Zhang, J.; Tang, Y.A.; Fu, Z.M.; Yang, Z.X.; Lu, Z.S. Repairing single and double atomic vacancies in a C3N monolayer with CO or NO molecules: A first-principles study. Phys. Chem. Chem. Phys. 2018, 20, 13517–13527. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.X.; Sun, W.; Hung, D.L.H.; Qiu, C.Y.; Makaremi, M.; Kumar, S.G.H.; Wan, L.L.; Ghoussoub, M.; Wood, T.E.; Xia, M.K.; et al. Catalytic CO2 reduction by palladium-decorated silicon–hydride nanosheets. Nat. Catal. 2019, 2, 46–54. [Google Scholar] [CrossRef]
- Cui, H.P.; Zheng, K.; Zhang, Y.Y.; Ye, H.Y.; Chen, X.P. Superior selectivity and sensitivity of C3N sensor in probing toxic gases NO2 and SO2. IEEE Electron Device Lett. 2018, 39, 284–287. [Google Scholar] [CrossRef]
- Ma, D.W.; Zhang, J.; Li, X.X.; He, C.Z.; Lu, Z.W.; Lu, Z.S.; Yang, Z.X.; Wang, Y.X. C3N monolayer as promising candidates for NO2 sensors. Sens. Actuator B Chem. 2018, 266, 664–673. [Google Scholar] [CrossRef]
- Babar, V.; Sharma, S.; Schwingenschlogl, U. Highly sensitive sensing of NO and NO2 gases by monolayer C3N. Adv. Theory Simul. 2018, 1, 1700008. [Google Scholar] [CrossRef]
- Eslami, M.; Moradi, M.; Moradi, R. DFT investigation of hydrogen adsorption on the C3N nanotube. Vacuum 2016, 133, 7–12. [Google Scholar] [CrossRef]
- Xiong, H.H.; Zhang, H.H.; Gan, L. A new bifunctional C3N nanosheet of NO2, SO2 gas sensor and CO2 separation: A first-principles study. Phys. E 2021, 126, 114463. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L.N.; Umar, A.; Chen, W.G.; Kumar, R. Pt nanoparticles decorated SnO2 nanoneedles for efficient CO gas sensing applications. Sens. Actuators B Chem. 2018, 256, 656–664. [Google Scholar] [CrossRef]
- Cui, H.; Chen, D.C.; Zhang, Y.; Zhang, X.X. Dissolved gas analysis in transformer oil using Pd catalyst decorated MoSe2 monolayer: A first-principles theory. Sustain. Mater. Technol. 2019, 20, e00094. [Google Scholar] [CrossRef]
- Liao, Y.M.; Peng, R.C.; Peng, S.D.; Zeng, W.; Zhou, Q. The adsorption of H2 and C2H2 on Ge-doped and Cr-doped graphene structures: A DFT study. Nanomaterial 2021, 11, 231. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Ma, S.X. Adsorption and sensing behaviors of Pd-doped InN monolayer upon CO and NO molecules: A first-principles study. Appl. Sci. 2019, 9, 3390. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.W.; Ju, W.W.; Li, T.X.; Zhang, X.W.; He, C.Z.; Ma, B.Y.; Lu, Z.S.; Yang, Z.X. The adsorption of CO and NO on the MoS2 monolayer doped with Au, Pt, Pd, or Ni: A first-principles study. Appl. Surf. Sci. 2016, 383, 98–105. [Google Scholar] [CrossRef]
- Capelle, K.; Gross, E.K.U. Spin-density functionals from current-density functional theory and vice versa: A road towards new approximations. Phys. Rev. Lett. 1997, 78, 1872–1875. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhou, Q.; Wang, J.X.; Xu, L.N.; Zeng, W. DFT Study on the Selective Adsorption Properties of Modified Graphene for SF6 Decompositions. IEEE Sens. J. 2021, 21, 3193–3200. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zeng, W.; Ye, H.; Li, Y.Q. Enhanced carbon monoxide sensing properties of TiO2 with exposed (001) facet: A combined first-principle and experimental study. Appl. Surf. Sci. 2018, 442, 507–516. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zeng, W.; Li, Y.Q. Computational study of surface orientation effect of rutile TiO2 on H2S and CO sensing mechanism. Appl. Surf. Sci. 2019, 495, 143619. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J.X.; Xu, L.N.; Zeng, W. Performance of intrinsic and modified graphene for the adsorption of H2S and CH4: A DFT study. Nanomaterial 2020, 10, 299. [Google Scholar] [CrossRef] [Green Version]
- Tkatchenko, A.; Distasio, R.A.; Head-Gordon, M.; Scheffler, M. Dispersion-corrected Moller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 094106. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.C.; Zhang, X.X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2and SO2F2: A DFT study. Appl. Phys. A Mater. Sci. Process. 2018, 124, 194. [Google Scholar] [CrossRef]
- Gui, X.X.; Zhou, Q.; Peng, S.D.; Xu, L.N.; Zeng, W. Dissolved gas analysis in transformer oil using Sb-doped graphene: A DFT study. Appl. Surf. Sci. 2020, 533, 147509. [Google Scholar] [CrossRef]
- Wang, Y.H.; Li, D.Y.; Lai, X.B.; Liu, B.Y.; Chen, Y.B.; Wang, F.P.; Wang, R.M.; Zhang, L.W. Direct observation of the hysteretic Fermi level modulation in monolayer MoS2 field effect transistors. Curr. Appl. Phys. 2020, 20, 293–303. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Zhang, J.; Tang, J. Adsorption behaviour of SF6 decomposed species onto Pd4-decorated single-walled CNT: A DFT study. Mol. Phys. 2018, 116, 1749–1755. [Google Scholar] [CrossRef]
- Wu, P.; Yin, N.Q.; Li, P.; Cheng, W.J.; Huang, M. The adsorption and diffusion behavior of noble metal adatoms (Pd, Pt, Cu, Ag and Au) on a MoS2 monolayer: A first-principles study. Phys. Chem. Chem. Phys. 2017, 19, 20713–20722. [Google Scholar] [CrossRef]
- Makaremi, M.; Mortazavi, B.; Singh, C.V. Adsorption of metallic, metalloidic, and nonmetallic adatoms on two-dimensional C3N. J. Phys. Chem. C 2017, 121, 18575–18583. [Google Scholar] [CrossRef] [Green Version]
- Hirshfeld, F.L. Bonded-atom fragments for describing molecular charge densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Liu, D.K.; Gui, Y.G.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X.X. Adsorption of SF6 decomposition components over Pd (1 1 1): A density functional theory study. Appl. Surf. Sci. 2019, 465, 172–179. [Google Scholar] [CrossRef]
- Lu, Z.S.; Yang, M.X.; Ma, D.W.; Lv, P.; Li, S.; Yang, Z.X. CO oxidation on Mn-N4 porphyrin-like carbon nanotube: A DFT-D study. Appl. Surf. Sci. 2017, 426, 1232–1240. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Chen, D.C.; Tang, J. Pt & Pd decorated CNT as a workable media for SOF2 sensing: A DFT study. Appl. Surf. Sci. 2018, 471, 335–341. [Google Scholar]
- Cui, H.; Zhang, X.X.; Zhang, G.Z.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Zhou, X.D.; Feng, W.X.; Guan, S.; Fu, B.T.; Su, W.Y.; Yao, Y.G. Computational characterization of monolayer C3N: A two-dimensional nitrogen-graphene crystal. J. Mater. Res. 2017, 32, 2993–3001. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.W.; Wang, Q.G.; Li, T.X.; He, C.Z.; Ma, B.Y.; Tang, Y.A.; Lu, Z.S.; Yang, Z.X. Repairing sulfur vacancies in the MoS2 monolayer by using CO, NO and NO2 molecules. J. Mater. Chem. C 2016, 4, 7093–7101. [Google Scholar] [CrossRef]
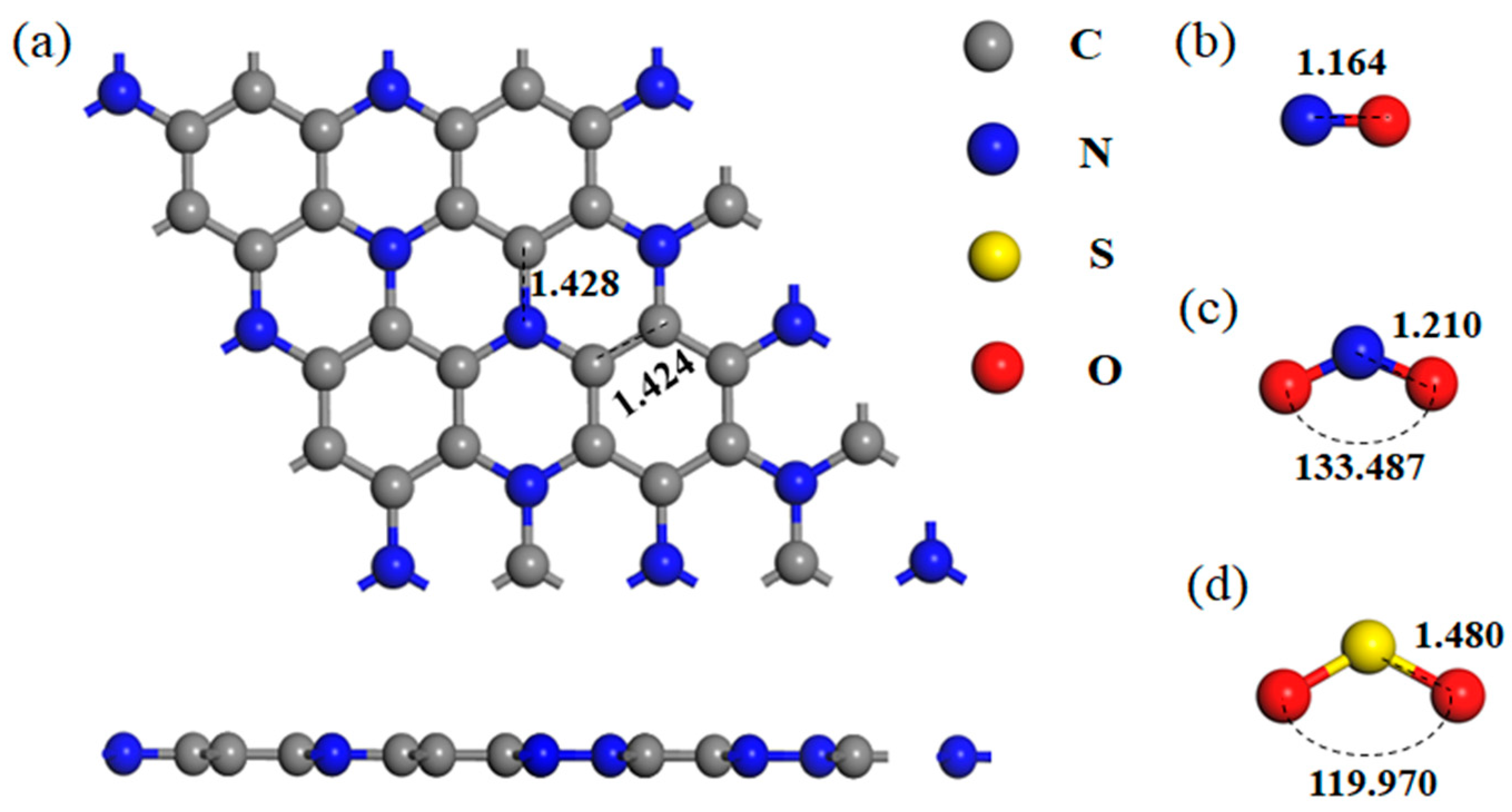



| Gas | Bond Length (Å) | Bond Angle (°) | ||
|---|---|---|---|---|
| NO | N-O | 1.164 | - | - |
| NO2 | N-O | 1.210 | O-N-O | 133.487 |
| SO2 | S-O | 1.480 | O-S-O | 119.970 |
| Gas | N | O | S |
|---|---|---|---|
| NO | 0.035 | −0.035 | - |
| NO2 | 0.375 | −0.188 | - |
| SO2 | - | 0.454 | −0.227 |
| System | The Length of Bond (Å) | Bond Angle (°) | Adsorption Distance (Å) | ||
|---|---|---|---|---|---|
| Pd-C3N + NO | N-O | 1.188 | - | 1.904 | |
| Pd-C | 2.046, 2.046, 2.050 | ||||
| Pd-C3N + NO2 | N-O | 1.281 | O-N-O | 111.674 | 2.202 |
| Pd-C | 1.978, 1.979, 2.016 | ||||
| Pd-C3N + SO2 | S-O | 1.495 | O-S-O | 119.932 | 2.261 |
| Pd-C | 2.046, 2.044, 1.990 | ||||
| System | Atom | Mulliken Charge (e) | QT (e) | Ead (eV) |
|---|---|---|---|---|
| Pd-C3N + NO | N | −0.033 | −0.122 | −1.83 |
| O | −0.089 | |||
| Pd-C3N + NO2 | N | 0.270 | −0.407 | −2.74 |
| O1 | −0.338 | |||
| O2 | −0.339 | |||
| Pd-C3N + SO2 | S | 0.457 | −0.177 | −1.61 |
| O1 | −0.303 | |||
| O2 | −0.331 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, R.; Zhou, Q.; Zeng, W. First-Principles Insight into Pd-Doped C3N Monolayer as a Promising Scavenger for NO, NO2 and SO2. Nanomaterials 2021, 11, 1267. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051267
Peng R, Zhou Q, Zeng W. First-Principles Insight into Pd-Doped C3N Monolayer as a Promising Scavenger for NO, NO2 and SO2. Nanomaterials. 2021; 11(5):1267. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051267
Chicago/Turabian StylePeng, Ruochen, Qu Zhou, and Wen Zeng. 2021. "First-Principles Insight into Pd-Doped C3N Monolayer as a Promising Scavenger for NO, NO2 and SO2" Nanomaterials 11, no. 5: 1267. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051267






