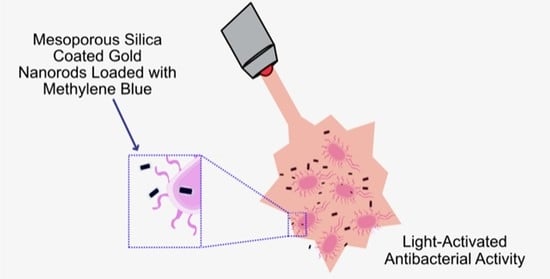
Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gold Nanorods
2.3. Preparation of AuNRs@Simes
2.4. Drug Loading
2.5. Freeze Drying of AuNRs@Simes-Drug
2.6. Antibacterial Activity
2.7. Statistics in Bacterial Samples
3. Results and Discussion
3.1. Synthesis of Gold Nanorods
3.2. Synthesis and Purification of AuNRs@Simes
3.3. Drug Loaded and Freeze-Drying Experiments
3.4. Exploring the Application of AuNRs@Simes-MB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet Chemical Synthesis of High Aspect Ratio Cylindrical Gold Nanorods. J. Phys. Chem. B 2001, 105, 4065–4067. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Lohse, S.E.; Murphy, C.J. The Quest for Shape Control: A History of Gold Nanorod Synthesis. Chem. Mater. 2013, 25, 1250–1261. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface Plasmon Resonance in Gold Nanoparticles: A Review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Plasmonic Photothermal Therapy (PPTT) Using Gold Nanoparticles. Lasers Med. Sci. 2008, 23, 217–228. [Google Scholar] [CrossRef]
- Liu, J.; Detrembleur, C.; De Pauw-Gillet, M.-C.; Mornet, S.; Jérôme, C.; Duguet, E. Gold Nanorods Coated with Mesoporous Silica Shell as Drug Delivery System for Remote Near Infrared Light-Activated Release and Potential Phototherapy. Small 2015, 11, 2323–2332. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, L.; Wang, J.; Jiang, X.; Li, X.; Hu, Z.; Ji, Y.; Wu, X.; Chen, C. Mesoporous Silica-Coated Gold Nanorods as a Light-Mediated Multifunctional Theranostic Platform for Cancer Treatment. Adv. Mater. 2012, 24, 1418–1423. [Google Scholar] [CrossRef]
- Kuo, W.-S.; Chang, C.-N.; Chang, Y.-T.; Yang, M.-H.; Chien, Y.-H.; Chen, S.-J.; Yeh, C.-S. Gold Nanorods in Photodynamic Therapy, as Hyperthermia Agents, and in Near-Infrared Optical Imaging. Angew. Chemie Int. Ed. 2010, 49, 2711–2715. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.; Yin, C.; Zhang, S.; Lin, G.; Li, Q. Surface Plasmon Enhanced Drug Efficacy Using Core-Shell Au@SiO2 Nanoparticle Carrier. Nanoscale 2013, 5, 3406–3411. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Murphy, C.J. Toxicity and Cellular Uptake of Gold Nanoparticles: What We Have Learned so Far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jiang, X.; Ji, Y.; Bai, R.; Zhao, Y.; Wu, X.; Chen, C. Surface Chemistry of Gold Nanorods: Origin of Cell Membrane Damage and Cytotoxicity. Nanoscale 2013, 5, 8384–8391. [Google Scholar] [CrossRef]
- Luo, G.-F.; Chen, W.-H.; Lei, Q.; Qiu, W.-X.; Liu, Y.-X.; Cheng, Y.-J.; Zhang, X.-Z. A Triple-Collaborative Strategy for High-Performance Tumor Therapy by Multifunctional Mesoporous Silica-Coated Gold Nanorods. Adv. Funct. Mater. 2016, 26, 4339–4350. [Google Scholar] [CrossRef]
- Xu, C.; Chen, F.; Valdovinos, H.F.; Jiang, D.; Goel, S.; Yu, B.; Sun, H.; Barnhart, T.E.; Moon, J.J.; Cai, W. Bacteria-like Mesoporous Silica-Coated Gold Nanorods for Positron Emission Tomography and Photoacoustic Imaging-Guided Chemo-Photothermal Combined Therapy. Biomaterials 2018, 165, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene Blue in Photodynamic Therapy: From Basic Mechanisms to Clinical Applications. Photodiagn. Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Ormond, A.; Freeman, H. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planas, O.; Bresolí-Obach, R.; Nos, J.; Gallavardin, T.; Ruiz-González, R.; Agut, M.; Nonell, S. Synthesis, Photophysical Characterization, and Photoinduced Antibacterial Activity of Methylene Blue-Loaded Amino- and Mannose-Targeted Mesoporous Silica Nanoparticles. Molecules 2015, 20, 6284–6298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-Y.; Wintner, A.; Seed, P.C.; Brauns, T.; Gelfand, J.A.; Hamblin, M.R. Antimicrobial Photodynamic Therapy Mediated by Methylene Blue and Potassium Iodide to Treat Urinary Tract Infection in a Female Rat Model. Sci. Rep. 2018, 8, 7257. [Google Scholar] [CrossRef]
- Parasuraman, P.; Anju, V.T.; Sruthil Lal, S.; Sharan, A.; Busi, S.; Kaviyarasu, K.; Arshad, M.; Dawoud, T.M.S.; Syed, A. Synthesis and Antimicrobial Photodynamic Effect of Methylene Blue Conjugated Carbon Nanotubes on E. Coli and S. Aureus. Photochem. Photobiol. Sci. 2019, 18, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-H.; Kim, B.-M.; Joe, A.; Han, H.-W.; Chen, X.; Cheng, Z.; Jang, E.-S. NIR-Light-Induced Surface-Enhanced Raman Scattering for Detection and Photothermal/Photodynamic Therapy of Cancer Cells Using Methylene Blue-Embedded Gold Nanorod@SiO2 Nanocomposites. Biomaterials 2014, 35, 3309–3318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, J.; Wu, G.; He, Y.; Zhang, L.; Yi, Y. Methylene Blue-Loaded Gold Nanobipyramids @SiO2 Enhanced Singlet Oxygen Generation for Phototherapy of Cancer Cells. Opt. Mater. Express 2017, 7, 409. [Google Scholar] [CrossRef]
- Li, Y.; Wen, T.; Zhao, R.; Liu, X.; Ji, T.; Wang, H.; Shi, X.; Shi, J.; Wei, J.; Zhao, Y.; et al. Localized Electric Field of Plasmonic Nanoplatform Enhanced Photodynamic Tumor Therapy. ACS Nano 2014, 8, 11529–11542. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cell Imaging and Photothermal Therapy in the Near-Infrared Region by Using Gold Nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.A.; Ali, M.R.K.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The Most Effective Gold Nanorod Size for Plasmonic Photothermal Therapy: Theory and in Vitro Experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Antibacterial Activity of Gold Nanorods against Staphylococcus Aureus and Propionibacterium Acnes: Misinterpretations and Artifacts. Int. J. Nanomed. 2017, 12, 7311–7322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.M.; Fouad, S.A.; Elshoky, H.A.; Mohammed, G.M.; Salaheldin, T.A. Antibacterial Effect of Gold Nanoparticles against Corynebacterium Pseudotuberculosis. Int. J. Vet. Sci. Med. 2017, 5, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, N.N.; Alkilany, A.M.; Khalil, E.A.; Al-Bakri, A.G. Nano-Photothermal Ablation Effect of Hydrophilic and Hydrophobic Functionalized Gold Nanorods on Staphylococcus Aureus and Propionibacterium Acnes. Sci. Rep. 2018, 8, 6881. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Wang, C.; Wang, X.; Li, L. Synthesis of Photothermal Nanocomposites and Their Application to Antibacterial Assays. Nanotechnology 2018, 29, 175601. [Google Scholar] [CrossRef]
- Khlebtsov, B.N.; Tuchina, E.S.; Khanadeev, V.A.; Panfilova, E.V.; Petrov, P.O.; Tuchin, V.V.; Khlebtsov, N.G. Enhanced Photoinactivation of Staphylococcus Aureus with Nanocomposites Containing Plasmonic Particles and Hematoporphyrin. J. Biophotonics 2013, 6, 338–351. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Scarabelli, L.; Sánchez-Iglesias, A.; Pérez-Juste, J.; Liz-Marzán, L.M. A “Tips and Tricks” Practical Guide to the Synthesis of Gold Nanorods. J. Phys. Chem. Lett. 2015, 6, 4270–4279. [Google Scholar] [CrossRef] [Green Version]
- Monem, A.S.; Elbialy, N.; Mohamed, N. Mesoporous Silica Coated Gold Nanorods Loaded Doxorubicin for Combined Chemo-Photothermal Therapy. Int. J. Pharm. 2014, 470, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cockerill, F.R.; Wiker, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- Pérez-Laguna, V.; Pérez-Artiaga, L.; Lampaya-Pérez, V.; López, S.C.; García-Luque, I.; Revillo, M.J.; Nonell, S.; Gilaberte, Y.; Rezusta, A. Comparative Effect of Photodynamic Therapy on Separated or Mixed Cultures of Streptococcus Mutans and Streptococcus Sanguinis. Photodiagn. Photodyn. Ther. 2017, 19, 98–102. [Google Scholar] [CrossRef]
- Wu, W.-C.; Tracy, J.B. Large-Scale Silica Overcoating of Gold Nanorods with Tunable Shell Thicknesses. Chem. Mater. 2015, 27, 2888–2894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, L.R.; Chapman, B.S.; Tracy, J.B. Understanding and Controlling the Morphology of Silica Shells on Gold Nanorods. Chem. Mater. 2018, 30, 6249–6258. [Google Scholar] [CrossRef]
- Huang, L.; Chen, X.; Li, Q. Synthesis of Microporous Molecular Sieves by Surfactant Decomposition. J. Mater. Chem. 2001, 11, 610–615. [Google Scholar] [CrossRef]
- Reinhardt, N.; Adumeau, L.; Lambert, O.; Ravaine, S.; Mornet, S. Quaternary Ammonium Groups Exposed at the Surface of Silica Nanoparticles Suitable for DNA Complexation in the Presence of Cationic Lipids. J. Phys. Chem. B 2015, 119, 6401–6411. [Google Scholar] [CrossRef]
- De Barros, H.R.; Piovan, L.; Sassaki, G.L.; de Araujo Sabry, D.; Mattoso, N.; Nunes, Á.M.; Meneghetti, M.R.; Riegel-Vidotti, I.C. Surface Interactions of Gold Nanorods and Polysaccharides: From Clusters to Individual Nanoparticles. Carbohydr. Polym. 2016, 152, 479–486. [Google Scholar] [CrossRef]
- Nithiyanantham, U.; Ozaydin, M.F.; Tazebay, A.S.; Kundu, S. Low Temperature Formation of Rectangular PbTe Nanocrystals and Their Thermoelectric Properties. New J. Chem. 2016, 40, 265–277. [Google Scholar] [CrossRef]
- Azarshin, S.; Moghadasi, J.; A Aboosadi, Z. Surface Functionalization of Silica Nanoparticles to Improve the Performance of Water Flooding in Oil Wet Reservoirs. Energy Explor. Exploit. 2017, 35, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Djafari, J.; Fernández-Lodeiro, C.; Fernández-Lodeiro, A.; Silva, V.; Poeta, P.; Igrejas, G.; Lodeiro, C.; Capelo, J.L.; Fernández-Lodeiro, J. Exploring the Control in Antibacterial Activity of Silver Triangular Nanoplates by Surface Coating Modulation. Front. Chem. 2019, 6, 1–11. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q.-H. Stable and Functionable Mesoporous Silica-Coated Gold Nanorods as Sensitive Localized Surface Plasmon Resonance (LSPR) Nanosensors. Langmuir 2009, 25, 9441–9446. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Ellis, L.-J.; Romer, I.; Tantra, R.; Carriere, M.; Allard, S.; Mayne-L’Hermite, M.; Minelli, C.; Unger, W.; Potthoff, A.; et al. Dispersion of Nanomaterials in Aqueous Media: Towards Procol Optimization. JoVE 2017, 130, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shenoi, R.A. Sugar-based systems. In Engineering of Biomaterials for Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 273–297. [Google Scholar]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Reis, S.; Sarmento, B. Facts and Evidences on the Lyophilization of Polymeric Nanoparticles for Drug Delivery. J. Control. Release 2016, 225, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Crowe, L.M.; Reid, D.S.; Crowe, J.H. Is Trehalose Special for Preserving Dry Biomaterials? Biophys. J. 1996, 71, 2087–2093. [Google Scholar] [CrossRef] [Green Version]
- Andreani, T.; Kiill, C.P.; de Souza, A.L.R.; Fangueiro, J.F.; Doktorovová, S.; Garcia, M.L.; Gramião, M.P.D.; Silva, A.M.; Souto, E.B. Effect of Cryoprotectants on the Reconstitution of Silica Nanoparticles Produced by Sol–Gel Technology. J. Therm. Anal. Calorim. 2015, 120, 1001–1007. [Google Scholar] [CrossRef]
- Sameti, M.; Bohr, G.; Ravi Kumar, M.N.V.; Kneuer, C.; Bakowsky, U.; Nacken, M.; Schmidt, H.; Lehr, C.-M. Stabilisation by Freeze-Drying of Cationically Modified Silica Nanoparticles for Gene Delivery. Int. J. Pharm. 2003, 266, 51–60. [Google Scholar] [CrossRef] [Green Version]
- Auer, G.K.; Weibel, D.B. Bacterial Cell Mechanics. Biochemistry 2017, 56, 3710–3724. [Google Scholar] [CrossRef]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular Mechanisms of Membrane Targeting Antibiotics. Biochim. Biophys. Acta 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Benaroudj, N.; Lee, D.H.; Goldberg, A.L. Trehalose Accumulation during Cellular Stress Protects Cells and Cellular Proteins from Damage by Oxygen Radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Waring, A.J.; Hong, M. Trehalose-Protected Lipid Membranes for Determining Membrane Protein Structure and Insertion. J. Magn. Reson. 2007, 184, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.; Debnath, K.; Jana, N.R.; Jana, N.R. Trehalose-Functionalized Gold Nanoparticle for Inhibiting Intracellular Protein Aggregation. Langmuir 2017, 33, 13996–14003. [Google Scholar] [CrossRef] [PubMed]
- Vanaporn, M.; Titball, R.W. Trehalose and Bacterial Virulence. Virulence 2020, 11, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, J.; Cesario, T.C.; Wang, X.; Yuan, J.S.; Rentzepis, P.M. Synergistic Reaction of Silver Nitrate, Silver Nanoparticles, and Methylene Blue against Bacteria. Proc. Natl. Acad. Sci. USA 2016, 113, 13612–13617. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Samples | E. coli | S. aureus | ||
|---|---|---|---|---|
| Log10CFU/mL | Log Reduction | Log10CFU/mL | Log Reduction | |
| I Control | 7.58 ± 0.20 (a) | - | 7.55 ± 0.11 (a, b) | - |
| NR Control | 7.70 ± 0.24 (a) | - | 7.51 ± 0.06 (a, b, c) | - |
| * AuNRs@Simes (1 mg/mL) | 7.88 ± 0.03 (a) | <1 | 6.70 ± 0.26 (e) | <1 |
| ** AuNRs@Simes (1 mg/mL) | 7.98 ± 0.09 (a) | <1 | 6.97 ± 0.13 (d, e) | <1 |
| * AuNRs@Simes-MB (1 mg/mL) | <1 | >7.58 ± 0.00 | <1 | >7.55 ± 0.00 |
| ** AuNRs@Simes-MB (1 mg/mL) | 7.66 ± 0.10 (a) | <1 | 7.13 ± 0.15 (c, d) | <1 |
| * MB (0.015 mg/mL) | <1 | >7.58 ± 0.00 | <1 | >7.55 ± 0.00 |
| ** MB (0.015 mg/mL) | 5.52 ± 0.21 (c) | 2.18 ± 0.21 | 7.20 ± 0.12 (b, c, d) | <1 |
| * TRH (10.34 mg/mL) | 7.86 ± 0.13 (a) | <1 | 7.95 ± 0.01 (a) | <1 |
| * MB (0.015 mg/mL) + TRH (10 mg/mL) | <1 | >7.58 ± 0.00 | <1 | >7.55 ± 0.00 |
| ** MB (0.015 mg/mL) + TRH (10 mg/mL) | 6.64 ± 0.07 (b) | 1.06 ± 0.07 | 7.20 ± 0.16 (b, c, d) | <1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lodeiro, A.; Djafari, J.; Fernández-Lodeiro, J.; Duarte, M.P.; Muchagato Mauricio, E.; Capelo-Martínez, J.L.; Lodeiro, C. Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications. Nanomaterials 2021, 11, 1338. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051338
Fernández-Lodeiro A, Djafari J, Fernández-Lodeiro J, Duarte MP, Muchagato Mauricio E, Capelo-Martínez JL, Lodeiro C. Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications. Nanomaterials. 2021; 11(5):1338. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051338
Chicago/Turabian StyleFernández-Lodeiro, Adrián, Jamila Djafari, Javier Fernández-Lodeiro, Maria Paula Duarte, Elisabete Muchagato Mauricio, José Luis Capelo-Martínez, and Carlos Lodeiro. 2021. "Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications" Nanomaterials 11, no. 5: 1338. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11051338