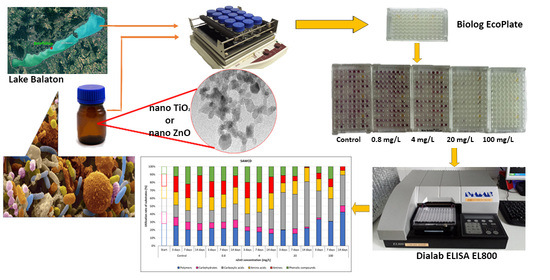
The Biolog EcoPlate™ Technique for Assessing the Effect of Metal Oxide Nanoparticles on Freshwater Microbial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Freshwater Samples from Lake Balaton
2.2. Characteristics of the Tested Nanomaterials
2.3. Experimental Setup
2.4. Monitoring
2.4.1. Physico-Chemical Methods
2.4.2. Microbiological Methods
2.4.3. Biolog EcoPlate™
2.4.4. Statistical Analysis of Data
3. Results
3.1. Physico-Chemical Characteristics of Lake Balaton
3.2. Effect of TiO2 and ZnO Nanoparticles on Enzymatic Activity
3.3. Effect of ZnO Nanoparticles on the Community-Level Physiological Profiles (CLPP)
3.4. Effect of TiO2 Nanoparticles on the Community-Level Physiological Profiles (CLPP)
4. Discussion
4.1. Assessment of the Impact of TiO2 Nanoparticles on Freshwater Microbial Diversity
4.2. Assessment of the Impact of ZnO Nanoparticles on Freshwater Microbial Diversity
4.3. Comparison of the Effects of TiO2 and ZnO Nanoparticles on Freshwater Microbial Diversity
4.4. Characterisation of the Applicability of the Biolog EcoPlate™ for the Evaluation of the Effects of Nanoparticles on Freshwater Microbial Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.; Naiman, R.J.; Knowler, D.J.; Le, C. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2015, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Bürgmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.H.; et al. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Zeglin, L.H. Stream microbial diversity in response to environmental changes: Review and synthesis of existing research. Front. microbiol. 2015, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [Green Version]
- Walters, C.; Pool, E. Nanotoxicology: A review. Toxicol.-New Asp. This Sci. Conundrum 2016, 10, 5772–6475. [Google Scholar]
- Mishra, S.; Keswani, C.; Abhilash, P.C.; Fraceto, L.F.; Singh, H.B. Integrated approach of Agri-Nanotechnology: Challenges and future trends. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kaegi, R.; Ulrich, A.; Sinnet, B.; Vonbank, R.; Wichser, A.; Zuleeg, S.; Simmler, H.; Brunner, S.; Vonmont, H.; Burkhardt, M.; et al. Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment. Environ. Pollut. 2008, 156, 233–239. [Google Scholar] [CrossRef]
- Kiser, M.A.; Westerhoff, P.; Benn, T.; Wang, Y.; Pérez-Rivera, J.; Hristovski, K. Titanium nanomaterial removal and release from wastewater treatment plants. Environ. Sci. Technol. 2009, 43, 6757–6763. [Google Scholar] [CrossRef]
- Limbach, L.K.; Bereiter, R.; Müller, E.; Krebs, R.; Gälli, R.; Stark, W.J. Removal of oxide nanoparticles in a model wastewater treatment plant: Influence of agglomeration and surfactants on clearing efficiency. Environ. Sci. Technol. 2008, 42, 5828–5833. [Google Scholar] [CrossRef]
- Farkas, J.; Peter, H.; Ciesielski, T.M.; Thomas, K.V.; Sommaruga, R.; Salvenmoser, W.; Weyhenmeyer, G.A.; Tranvik, L.J.; Jenssen, B.M. Impact of TiO2 nanoparticles on freshwater bacteria from three Swedish lakes. Sci. Total Environ. 2015, 535, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Nowack, B.; Mueller, N.C. Exposure modeling of engineered nanoparticles in the environment. EMPA Act. 2008, 41, 63. [Google Scholar]
- Battin, T.J.; Kammer, F.V.D.; Weilhartner, A.; Ottofuelling, S.; Hofmann, T. Nanostructured TiO2: Transport behavior and effects on aquatic microbial communities under environmental conditions. Environ. Sci. Technol. 2009, 43, 8098–8104. [Google Scholar] [CrossRef]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef]
- Londono, N.; Donovan, A.R.; Shi, H.; Geisler, M.; Liang, Y. Impact of TiO2 and ZnO nanoparticles on an aquatic microbial community: Effect at environmentally relevant concentrations. Nanotoxicology 2017, 11, 1140–1156. [Google Scholar] [CrossRef]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Zhao, L.; Lopez-Moreno, M.L.; de la Rosa, G.; Hong, J.; Gardea-Torresdey, J.L. Nanomaterials and the environment: A review for the biennium 2008–2010. J. Hazard. Mater. 2011, 186, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Klaine, S.J.; Alvarez, P.J.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851. [Google Scholar] [CrossRef] [PubMed]
- Frenk, S.; Ben-Moshe, T.; Dror, I.; Berkowitz, B.; Minz, D. Effect of metal oxide nanoparticles on microbial community structure and function in two different soil types. PLoS ONE 2013, 8, e84441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baysal, A.; Saygin, H.; Ustabasi, G.S. Physicochemical transformation of ZnO and TiO2 nanoparticles in sea water and its impact on bacterial toxicity. Environ. Health Eng. Manag. 2019, 6, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Fekete-Kertész, I.; Piszmán, D.; Molnár, M. Particle Size and Concentration Dependent Ecotoxicity of Nano- and Microscale TiO2—Comparative Study by Different Aquatic Test Organisms of Different Trophic Levels. Water. Air. Soil Pollut. 2017, 228, 245. [Google Scholar] [CrossRef]
- Parada, J.; Rubilar, O.; Fernández-Baldo, M.A.; Bertolino, F.A.; Durán, N.; Seabra, A.B.; Tortella, G.R. The nanotechnology among US: Are metal and metal oxides nanoparticles a nano or mega risk for soil microbial communities? Crit. Rev. Biotechnol. 2019, 39, 157–172. [Google Scholar] [CrossRef]
- Barnes, R.J.; van der Gast, C.J.; Riba, O.; Lehtovirta, L.E.; Prosser, J.I.; Dobson, P.J.; Thompson, I.P. The impact of zero-valent iron nanoparticles on a river water bacterial community. J. Hazard. Mater. 2010, 184, 73–80. [Google Scholar] [CrossRef]
- Echavarri-Bravo, V.; Paterson, L.; Aspray, T.J.; Porter, J.S.; Winson, M.K.; Thornton, B.; Hartl, M.G.J. Shifts in the metabolic function of a benthic estuarine microbial community following a single pulse exposure to silver nanoparticles. Environ. Pollut. 2015, 201, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Zabetakis, K.M.; De Guzmán, G.T.N.; Torrents, A.; Yarwood, S. Toxicity of zero-valent iron nanoparticles to a trichloroethylene-degrading groundwater microbial community. J. Environ. Sci. Health Part A Toxic/Hazardous Subst. Environ. Eng. 2015, 50, 794–805. [Google Scholar] [CrossRef]
- Christian, B.W.; Lind, O.T. Key issues concerning Biolog use for aerobic and anaerobic freshwater bacterial community-level physiological profiling. Int. Rev. Hydrobiol. 2006, 91, 257–268. [Google Scholar] [CrossRef]
- Xie, G.; Tang, X.; Shao, K.; Zhu, G.; Gao, G. Ecotoxicology and Environmental Safety Bacterial diversity, community composition and metabolic function in Lake Tianmuhu and its dammed river: Effects of domestic wastewater and damming. Ecotoxicol. Environ. Saf. 2021, 213, 112069. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Nga, N.T.H.; Ngoc, T.T.B.; Trinh, N.T.M.; Thuoc, T.L.; Thao, D.T.P. Optimization and application of MTT assay in determining density of suspension cells. Anal. Biochem. 2020, 610, 113937. [Google Scholar] [CrossRef]
- Ekennia, A.; Uduagwu, D.; Olowu, O.; Nwanji, O.; Oje, O.; Daniel, B.; Mgbii, S.; Emma-Uba, C. Biosynthesis of zinc oxide nanoparticles using leaf extracts of Alchornea laxiflora and its tyrosinase inhibition and catalytic studies. Micron 2021, 141, 102964. [Google Scholar] [CrossRef]
- Garland, J.L. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol. Ecol. 1997, 24, 289–300. [Google Scholar] [CrossRef]
- Stefanowicz, A. The biolog plates technique as a tool in ecological studies of microbial communities. Polish J. Environ. Stud. 2006, 15, 669–676. [Google Scholar]
- Kenarova, A.; Radeva, G.; Traykov, I.; Boteva, S. Community level physiological profiles of bacterial communities inhabiting uranium mining impacted sites. Ecotoxicol. Environ. Saf. 2014, 100, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Muńiz, S.; Lacarta, J.; Pata, M.P.; Jiménez, J.J.; Navarro, E. Analysis of the diversity of substrate utilisation of soil bacteria exposed to Cd and earthworm activity using generalised additive models. PLoS ONE 2014, 9, e85057. [Google Scholar] [CrossRef] [PubMed]
- Gryta, A.; Frąc, M.; Oszust, K. The Application of the Biolog EcoPlate Approach in Ecotoxicological Evaluation of Dairy Sewage Sludge. Appl. Biochem. Biotechnol. 2014, 174, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Garau, G.; Castaldi, P.; Santona, L.; Deiana, P.; Melis, P. Influence of red mud, zeolite and lime on heavy metal immobilization, culturable heterotrophic microbial populations and enzyme activities in a contaminated soil. Geoderma 2007, 142, 47–57. [Google Scholar] [CrossRef]
- Magdolna Nagy, Z.; Gruiz, K.; Molnár, M.; Fenyvesi, É. Comparative evaluation of microbial and chemical methods for assessing 4-chlorophenol biodegradation in soil. Period. Polytech. Chem. Eng. 2013, 57, 25–35. [Google Scholar] [CrossRef]
- Feigl, V.; Ujaczki, É.; Vaszita, E.; Molnár, M. Influence of red mud on soil microbial communities: Application and comprehensive evaluation of the Biolog EcoPlate approach as a tool in soil microbiological studies. Sci. Total Environ. 2017, 595, 903–911. [Google Scholar] [CrossRef] [Green Version]
- Frąc, M.; Oszust, K.; Lipiec, J. Community level physiological profiles (CLPP), characterization and microbial activity of soil amended with dairy sewage sludge. Sensors 2012, 12, 3253–3268. [Google Scholar] [CrossRef] [Green Version]
- Sala, M.M.; Arrieta, J.M.; Boras, J.A.; Duarte, C.M.; Vaqué, D. The impact of ice melting on bacterioplankton in the Arctic Ocean. Polar Biol. 2010, 33, 1683–1694. [Google Scholar] [CrossRef] [Green Version]
- Farkas, É.; Feigl, V.; Gruiz, K.; Vaszita, E.; Fekete-Kertész, I.; Tolner, M.; Kerekes, I.; Pusztai, É.; Kari, A.; Uzinger, N.; et al. Long-term effects of grain husk and paper fibre sludge biochar on acidic and calcareous sandy soils—A scale-up field experiment applying a complex monitoring toolkit. Sci. Total Environ. 2020, 731, 138988. [Google Scholar] [CrossRef] [PubMed]
- Jałowiecki, Ł.; Chojniak, J.M.; Dorgeloh, E.; Hegedusova, B.; Ejhed, H.; Magnér, J.; Płaza, G.A. Microbial community profiles in wastewaters from onsite wastewater treatment systems technology. PLoS ONE 2016, 11, e147725. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Tam, L.; Derry, A.M.; Kevan, P.G.; Trevors, J.T. Functional diversity and community structure of microorganisms in rhizosphere and non-rhizosphere Canadian arctic soils. Biodivers. Conserv. 2001, 10, 1933–1947. [Google Scholar] [CrossRef]
- Moore, J.C. Diversity, Taxonomic versus Functional. In Encyclopedia of Biodiversity, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2013; Volume 2, pp. 648–656. [Google Scholar] [CrossRef]
- Manjunath, M.; Kumar, U.; Yadava, R.B.; Rai, A.B.; Singh, B. Influence of organic and inorganic sources of nutrients on the functional diversity of microbial communities in the vegetable cropping system of the Indo-Gangetic plains. Comptes Rendus-Biol. 2018, 341, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Preston-Mafham, J.; Boddy, L.; Randerson, P.F. Analysis of microbial community functional diversity using sole-carbon-source utilisation profiles—A critique. FEMS Microbiol. Ecol. 2006, 42, 1–14. [Google Scholar] [CrossRef]
- Miao, L.; Wang, P.; Wang, C.; Hou, J.; Yao, Y.; Liu, J.; Lv, B.; Yang, Y.; You, G.; Xu, Y.; et al. Effect of TiO2 and CeO2 nanoparticles on the metabolic activity of surficial sediment microbial communities based on oxygen microelectrodes and high-throughput sequencing. Water Res. 2018, 129, 287–296. [Google Scholar] [CrossRef]
- Chavan, S.; Nadanathangam, V. Shifts in metabolic patterns of soil bacterial communities on exposure to metal engineered nanomaterials. Ecotoxicol. Environ. Saf. 2020, 189, 110012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Oberdörster, G.; Biswas, P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J. Nanopart. Res. 2009, 11, 77–89. [Google Scholar] [CrossRef]
- Jiang, J.; Oberdörster, G.; Elder, A.; Gelein, R.; Mercer, P.; Biswas, P. Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2008, 2, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Casas-Beltran, D.A.; Hernández-Pedraza, M.; Alvarado-Flores, J. Estimation of the discharge of sunscreens in aquatic environments of the Mexican Caribbean. Environments 2020, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Zhang, Y.; Guo, R.; Meng, F.; Gao, Y.; Ma, C.; Zhang, H. Harmful effect of nanoparticles on the functions of freshwater ecosystems: Insight into nanoZnO-polluted stream. Chemosphere 2019, 214, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Y.; Yin, Y.; Zhang, J.; Ma, H.; Li, K.; Wan, N. Do environmental concentrations of zinc oxide nanoparticle pose ecotoxicological risk to aquatic fungi associated with leaf litter decomposition? Water Res. 2020, 178, 115840. [Google Scholar] [CrossRef] [PubMed]
- Kusi, J.; Scheuerman, P.R.; Maier, K.J. Emerging environmental contaminants (silver nanoparticles) altered the catabolic capability and metabolic fingerprinting of microbial communities. Aquat. Toxicol. 2020, 228, 105633. [Google Scholar] [CrossRef] [PubMed]
- Zhen, T.; Fan, W.; Wang, H.; Cao, X.; Xu, X. Monitoring Soil Microorganisms with Community-Level Physiological Profiles Using Biolog EcoPlates™ in Chaohu Lakeside Wetland, East China. Eurasian Soil Sci. 2020, 53, 1142–1153. [Google Scholar] [CrossRef]
- Melita, M.; Amalfitano, S.; Preziosi, E.; Ghergo, S.; Frollini, E.; Parrone, D.; Zoppini, A. Physiological profiling and functional diversity of groundwater microbial communities in a municipal solid waste landfill area. Water 2019, 11, 2624. [Google Scholar] [CrossRef] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Harch, B.D.; Correll, R.L.; Meech, W.; Kirkby, C.A.; Pankhurst, C.E. Using the Gini coefficient with BIOLOG substrate utilisation data to provide an alternative quantitative measure for comparing bacterial soil communities. J. Microbiol. Methods 1997, 30, 91–101. [Google Scholar] [CrossRef]
- Adams, T.C.; Brye, K.R.; Savin, M.C.; Lee, J.A.; Gbur, E.E. Microbial carbon substrate utilization differences among high- and average-yield soybean areas. Agriculture 2017, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- DeJong, T.M. A Comparison of Three Diversity Indices Based on Their Components of Richness and Evenness. Oikos 1975, 26, 222–227. [Google Scholar] [CrossRef]
- Sutcliffe, B.; Hose, G.C.; Harford, A.J.; Midgley, D.J.; Greenfield, P.; Paulsen, I.T.; Chariton, A.A. Microbial communities are sensitive indicators for freshwater sediment copper contamination. Environ. Pollut. 2019, 247, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- El Fantroussi, S.; Verschuere, L.; Verstraete, W.; Top, E.M. Effect of phenylurea herbicides on soil microbial communities estimated by analysis of 16S rRNA gene fingerprints and community-level physiological profiles. Appl. Environ. Microbiol. 1999, 65, 982–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ros, M.; Goberna, M.; Pascual, J.A.; Klammer, S.; Insam, H. 16S rDNA analysis reveals low microbial diversity in community level physiological profile assays. J. Microbiol. Methods 2008, 72, 221–226. [Google Scholar] [CrossRef]
- Lladó, S.; Baldrian, P. Community-level physiological profiling analyses show potential to identify the copiotrophic bacteria present in soil environments. PLoS ONE 2017, 12, e171638. [Google Scholar] [CrossRef] [PubMed]














| Samples from Lake Balaton | pH (1) | EC (2) | NO3 (NO2)-N (3) | K+ (4) | Na+ (4) | Ca2+ (4) | Mg2+ (4) | Cl- (5) | CO32- (6) | HCO3- (6) | SO42- (7) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| - | µS/cm | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | |
| Sample collected in July | 8.3 ±0.2 | 707 ±16 | 0.22 ±0.10 | 6.7 ±0.2 | 36.4 ±0.4 | 33.9 ± | 58.8 ±0.1 | 38.4 ±2.6 | 27.9 ±13.2 | 233.4 ±26.8 | 134.2 ±0.2 |
| Sample collected in October | 8.8 ±0.0 | 664 ±20 | 0.14 ±0.00 | 7.2 ±0.6 | 37.4 ±1.3 | 27.6 ±2.7 | 60.3 ±2.1 | 40.3 ±0.0 | 46.5 ±4.4 | 211.3 ±40.1 | 141.5 ±4.6 |
| Source of Variation | d.f. | Mean Square | F Ratio | p-Value | Source of Variation | d.f. | Mean Square | F Ratio | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| AWCD | SR | ||||||||
| Treatment | 4 | 0.55 | 3317.68 | 0.000 | Treatment | 4 | 536.02 | 989.57 | 0.000 |
| Time | 2 | 0.01 | 7.95 | 0.041 | Time | 2 | 9.95 | 23.88 | 0.000 |
| Time × Treatment | 8 | 0.00 | 1.96 | 0.269 | Time × Treatment | 8 | 3.14 | 7.52 | 0.000 |
| AUC | H | ||||||||
| Treatment | 4 | 3.16 | 3299.60 | 0.001 | Treatment | 4 | 1.22 | 1487.4 | 0.000 |
| Time | 2 | 0.15 | 117.61 | 0.000 | Time | 2 | 0.18 | 25.2 | 0.000 |
| Time × Treatment | 8 | 0.03 | 19.46 | 0.006 | Time × Treatment | 8 | 0.10 | 13.5 | 0.000 |
| Source of variation | d.f. | Mean square | F ratio | p-value | Source of variation | d.f. | Mean square | F ratio | p-value |
| E | U | ||||||||
| Treatment | 3 | 3.38 | 223.07 | 0.000 | Treatment | 4 | 31.38 | 1230.25 | 0.000 |
| Time | 2 | 0.04 | 0.69 | 0.526 | Time | 2 | 0.70 | 0.70 | 0.000 |
| Time × Treatment | 6 | 0.05 | 0.78 | 0.606 | Time × Treatment | 8 | 0.23 | 0.23 | 0.000 |
| D | G | ||||||||
| Treatment | 4 | 0.02 | 50.87 | 0.000 | Treatment | 4 | 4.29 | 287.48 | 0.000 |
| Time | 2 | 0.01 | 30.71 | 0.000 | Time | 2 | 0.04 | 1.24 | 0.339 |
| Time × Treatment | 8 | 0.00 | 11.49 | 0.000 | Time × Treatment | 8 | 0.24 | 7.31 | 0.005 |
| Source of Variation | d.f. | Mean Square | F Ratio | p-Value | Source of Variation | d.f. | Mean Square | F Ratio | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| AWCD | SR | ||||||||
| Treatment | 4 | 0.01 | 7.01 | 0.028 | Treatment | 4 | 5.67 | 4.166 | 0.059 |
| Time | 2 | 0.04 | 52.87 | 0.000 | Time | 2 | 21.30 | 26.438 | 0.000 |
| Time × Treatment | 8 | 0.00 | 4.49 | 0.015 | Time × Treatment | 8 | 5.14 | 6.381 | 0.002 |
| AUC | H | ||||||||
| Treatment | 4 | 0.25 | 35.38 | 0.000 | Treatment | 4 | 0.01 | 6.4 | 0.034 |
| Time | 2 | 0.24 | 53.20 | 0.000 | Time | 2 | 0.05 | 40.9 | 0.000 |
| Time × Treatment | 8 | 0.04 | 9.60 | 0.000 | Time × Treatment | 8 | 0.00 | 2.1 | 0.132 |
| Source of variation | d.f. | Mean square | F ratio | p-value | Source of variation | d.f. | Mean square | F ratio | p-value |
| E | U | ||||||||
| Treatment | 4 | 0.00 | 5.4 | 0.046 | Treatment | 4 | 0.34 | 6.80 | 0.030 |
| Time | 2 | 0.00 | 2.7 | 0.114 | Time | 2 | 1.26 | 107.30 | 0.000 |
| Time × Treatment | 8 | 0.00 | 4.3 | 0.018 | Time × Treatment | 8 | 0.10 | 8.75 | 0.001 |
| D | G | ||||||||
| Treatment | 4 | 0.00 | 2 | 0.239 | Treatment | 4 | 0.01 | 7.426 | 0.025 |
| Time | 2 | 0.00 | 46 | 0.000 | Time | 2 | 0.03 | 122.266 | 0.000 |
| Time × Treatment | 8 | 0.00 | 2 | 0.103 | Time × Treatment | 8 | 0.00 | 4.887 | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, I.; Molnár, S.; Vaszita, E.; Molnár, M. The Biolog EcoPlate™ Technique for Assessing the Effect of Metal Oxide Nanoparticles on Freshwater Microbial Communities. Nanomaterials 2021, 11, 1777. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11071777
Németh I, Molnár S, Vaszita E, Molnár M. The Biolog EcoPlate™ Technique for Assessing the Effect of Metal Oxide Nanoparticles on Freshwater Microbial Communities. Nanomaterials. 2021; 11(7):1777. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11071777
Chicago/Turabian StyleNémeth, Imre, Szabina Molnár, Emese Vaszita, and Mónika Molnár. 2021. "The Biolog EcoPlate™ Technique for Assessing the Effect of Metal Oxide Nanoparticles on Freshwater Microbial Communities" Nanomaterials 11, no. 7: 1777. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11071777