Breaking Down SERS Detection Limit: Engineering of a Nanoporous Platform for High Sensing and Technology
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
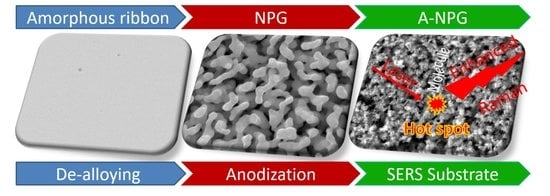
3.1. Morphology Evolution during Anodization Treatment
3.2. Mechanical Stability and Electrocatalytic Properties
3.3. SERS Activity
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ye, X.-L.; Jin, H.-J. Sealing-Free Fast-Response Paraffin/Nanoporous Gold Hybrid Actuator. Nanotechnology 2017, 28, 385501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Liu, X.; Wang, H.; Wu, Y.; Lu, Z.P. Development of Electrochemical Supercapacitors with Uniform Nanoporous Silver Network. Electrochim. Acta 2015, 182, 224–229. [Google Scholar] [CrossRef]
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic Origins of the High Catalytic Activity of Nanoporous Gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Scaglione, F.; Fiore, G.; Celegato, F.; Rizzi, P. Nanostructured Molybdenum Oxides from Aluminium-Based Intermetallic Compound: Synthesis and Application in Hydrogen Evolution Reaction. Nanomaterials 2021, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Alladio, E.; Damin, A.; Turci, F.; Baggiani, C.; Giovannoli, C.; Bordiga, S.; Battezzati, L.; Rizzi, P. Functionalized Nanoporous Gold as a New Biosensor Platform for Ultra-Low Quantitative Detection of Human Serum Albumin. Sens. Actuators B Chem. 2019, 288, 460–468. [Google Scholar] [CrossRef]
- Yang, C.L.; Zhang, X.H.; Lan, G.; Chen, L.Y.; Chen, M.W.; Zeng, Y.Q.; Jiang, J.Q. Pd-Based Nanoporous Metals for Enzyme-Free Electrochemical Glucose Sensors. Chin. Chem. Lett. 2014, 25, 496–500. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, S.; Shi, P.; Huang, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Denis, P.; Fecht, H.J. Nanoporous Gold Chemically De-Alloyed from Au-Based Amorphous Thin Film for Electrochemical Nonenzymatic H 2 O 2 Sensing. Chem. Phys. Lett. 2019, 723, 22–27. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L. Improving the Chemical De-Alloying of Amorphous Au Alloys. Corros. Sci. 2017, 127, 141–146. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Celegato, F.; Denis, P.; Fecht, H.-J.; Rizzi, P.; Battezzati, L. Shape Controlled Gold Nanostructures on De-Alloyed Nanoporous Gold with Excellent SERS Performance. Chem. Phys. Lett. 2018, 709, 46–51. [Google Scholar] [CrossRef]
- Rizzi, P.; Scaglione, F.; Battezzati, L. Nanoporous Gold by Dealloying of an Amorphous Precursor. J. Alloys Compd. 2014, 586, S117–S120. [Google Scholar] [CrossRef]
- Van Petegem, S.; Brandstetter, S.; Maass, R.; Hodge, A.M.; El-Dasher, B.S.; Biener, J.; Schmitt, B.; Borca, C.; Swygenhoven, H. Van on the Microstructure of Nanoporous Gold: An X-Ray Diffraction Study. Nano Lett. 2009, 9, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ding, Y.; Xu, C.; Inoue, A.; Sakurai, T.; Chen, M. Nanoporous Metals by Dealloying Multicomponent Metallic Glasses. Chem. Mater. 2008, 20, 4548–4550. [Google Scholar] [CrossRef]
- Paschalidou, E.M.; Celegato, F.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Gebert, A.; Oswald, S.; Wolff, U.; Mihaylov, L.; Spassov, T. The Mechanism of Generating Nanoporous Au by De-Alloying Amorphous Alloys. Acta Mater. 2016, 119, 177–183. [Google Scholar] [CrossRef]
- Scaglione, F.; Celegato, F.; Rizzi, P.; Battezzati, L. A Comparison of De-Alloying Crystalline and Amorphous Multicomponent Au Alloys. Intermetallics 2015, 66, 82–87. [Google Scholar] [CrossRef]
- Scaglione, F.; Xue, Y.; Celegato, F.; Rizzi, P.; Battezzati, L. Amorphous Molybdenum Sulphide @ Nanoporous Gold as Catalyst for Hydrogen Evolution Reaction in Acidic Environment. J. Mater. Sci. 2018, 53, 12388–12398. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Denis, P.; Fecht, H.-J. Electrodeposited Platinum on De-Alloyed Nanoporous Gold with Enhanced Electro-Catalytic Performance. Appl. Surf. Sci. 2019, 476, 412–417. [Google Scholar] [CrossRef]
- Paschalidou, E.M.; Scaglione, F.; Gebert, A.; Oswald, S.; Rizzi, P.; Battezzati, L. Partially and Fully De-Alloyed Glassy Ribbons Based on Au: Application in Methanol Electro-Oxidation Studies. J. Alloys Compd. 2016, 667, 302–309. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M. Nanoporous Metals for Catalytic and Optical Applications. MRS Bull. 2009, 34, 569–576. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, Applications, and the Future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem.-Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, H.; Zhao, G.; Tatsuma, T. Shape-Controlled Electrodeposition of Gold Nanostructures. J. Phys. Chem. B 2006, 110, 23478–23481. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Z.; Yang, Y.; Zhang, Z. Arrays of Aligned, Single Crystalline Silver Nanorods for Trace Amount Detection. J. Phys. D Appl. Phys. 2008, 41, 152007. [Google Scholar] [CrossRef]
- Chalmers, J.M.; Griffiths, P.R. Handbook of Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2001; Volume 1. [Google Scholar] [CrossRef]
- Morton, S.M.; Silverstein, D.W.; Jensen, L. Theoretical Studies of Plasmonics Using Electronic Structure Methods. Chem. Rev. 2011, 111, 3962–3994. [Google Scholar] [CrossRef] [PubMed]
- Pieczonka, N.P.W.; Aroca, R.F. Single Molecule Analysis by Surfaced-Enhanced Raman Scattering. Chem. Soc. Rev. 2008, 37, 946–954. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Chen, L.; Guan, P.; Chen, B.; Fujita, T.; Yamaguchi, Y.; Iwasaki, H.; Xue, Q.-K.; Chen, M. Large-Scale Growth of Sharp Gold Nano-Cones for Single-Molecule SERS Detection. RSC Adv. 2016, 6, 2882–2887. [Google Scholar] [CrossRef]
- Lim, D.-K.; Jeon, K.-S.; Hwang, J.-H.; Kim, H.; Kwon, S.; Suh, Y.D.; Nam, J.-M. Highly Uniform and Reproducible Surface-Enhanced Raman Scattering from DNA-Tailorable Nanoparticles with 1-Nm Interior Gap. Nat. Nanotechnol. 2011, 6, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Paschalidou, E.M.; Rizzi, P.; Bordiga, S.; Battezzati, L. Nanoporous Gold Obtained from a Metallic Glass Precursor Used as Substrate for Surface-Enhanced Raman Scattering. Philos. Mag. Lett. 2015, 95, 474–482. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L. High Performance SERS on Nanoporous Gold Substrates Synthesized by Chemical De-Alloying a Au-Based Metallic Glass. Appl. Surf. Sci. 2017, 426, 1113–1120. [Google Scholar] [CrossRef]
- Scaglione, F.; Rizzi, P.; Celegato, F.; Battezzati, L. Synthesis of Nanoporous Gold by Free Corrosion of an Amorphous Precursor. J. Alloys Compd. 2014, 615, S142–S147. [Google Scholar] [CrossRef]
- Xiao, S.; Xiao, F.; Hu, Y.; Yuan, S.; Wang, S.; Qian, L.; Liu, Y. Hierarchical Nanoporous Gold-Platinum with Heterogeneous Interfaces for Methanol Electrooxidation. Sci. Rep. 2014, 4, 4370. [Google Scholar] [CrossRef] [Green Version]
- Lukaszewski, M.; Soszko, M.; Czerwiński, A. Electrochemical Methods of Real Surface Area Determination of Noble Metal Electrodes—An Overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Liu, H.; Hou, Y.; Hirata, A.; Fujita, T.; Chen, M. Effect of Residual Silver on Surface-Enhanced Raman Scattering of Dealloyed Nanoporous Gold. J. Phys. Chem. C 2011, 115, 19583–19587. [Google Scholar] [CrossRef]
- Nishio, K.; Masuda, H. Anodization of Gold in Oxalate Solution to Form a Nanoporous Black Film. Angew. Chem.-Int. Ed. 2011, 50, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yao, Y.; Wang, P.; Yang, Y.; Xia, Y.; Liu, J.; Li, Z.; Huang, W. Anodic Fabrication of Nanoporous Gold Films from Pure Gold in Oxalic Acid Solution and Their Applications in Electrocatalysis and Sers. Int. J. Electrochem. Sci. 2013, 8, 1863–1870. [Google Scholar]
- Ocean Optics SERS Substrates Surface Enhanced Raman Spectroscopy. Available online: www.oceanoptics.com (accessed on 20 March 2020).
- Hamamatsu SERS Substrate That Enhances the Weak Raman Scattered Light from the Molecu. Available online: https://www.hamamatsu.com/jp/en/product/type/J12853/index.html (accessed on 20 March 2020).
- Ngai, K.S.; Tan, W.T.; Zainal, Z.; Zawawi, R.B.M.; Zidan, M. Electrochemical Oxidation of Ascorbic Acid Mediated by Single-Walled Carbon Nanotube/Tungsten Oxide Nanoparticles Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2012, 7, 4210–4222. [Google Scholar]
- Joo, S.W. Surface-Enhanced Raman Scattering of 4,4′-Bipyridine on Gold Nanoparticle Surfaces. Vib. Spectrosc. 2004, 34, 269–272. [Google Scholar] [CrossRef]
- Suzuki, M.; Niidome, Y.; Yamada, S. Adsorption Characteristics of 4,4′-Bipyridine Molecules on Gold Nanosphere Films Studied by Surface-Enhanced Raman Scattering. Thin Solid Film. 2006, 496, 740–747. [Google Scholar] [CrossRef]
- Huang, J.; Chen, F.; Zhang, Q.; Zhan, Y.; Ma, D.; Xu, K.; Zhao, Y. 3D Silver Nanoparticles Decorated Zinc Oxide/Silicon Heterostructured Nanomace Arrays as High-Performance Surface-Enhanced Raman Scattering Substrates. ACS Appl. Mater. Interfaces 2015, 7, 5725–5735. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, X.; Dai, Z.; Wu, W.; Zhang, X.; Fu, L.; Jiang, C. Ultrasensitive SERS Performance in 3D “Sunflower-like” Nanoarrays Decorated with Ag Nanoparticles. Nanoscale 2017, 9, 3114–3120. [Google Scholar] [CrossRef]
- Zhang, L.; Lang, X.; Hirata, A.; Chen, M. Wrinkled Nanoporous Gold Films with Ultrahigh Surface-Enhanced Raman Scattering Enhancement. ACS Nano 2011, 5, 4407–4413. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Paschalidou, E.M.; Rizzi, P.; Battezzati, L. Excellent Surface Enhanced Raman Scattering Obtained with Nanoporous Gold Fabricated by Chemical De-Alloying. Chem. Phys. Lett. 2016, 665, 6–9. [Google Scholar] [CrossRef]
- Federico, S.; Rizzi, P.; Battezzati, L. METODO PER LA PREPARAZIONE DI ORO NANOPOROSO ANODIZZATO, ORO NANOPOROSO ANODIZZATO E SUOI. USI. Patent number 102020000024382, 15 October 2020. [Google Scholar]
- Federico, S.; Rizzi, P.; Battezzati, L. METODO PER LA PREPARAZIONE DI ORO NANOPOROSO ANODIZZATO, ORO NANOPOROSO ANODIZZATO E SUOI. USI. Patent number PCT/IB2021/059525, 15 October 2021. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaglione, F.; Battezzati, L.; Rizzi, P. Breaking Down SERS Detection Limit: Engineering of a Nanoporous Platform for High Sensing and Technology. Nanomaterials 2022, 12, 1737. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12101737
Scaglione F, Battezzati L, Rizzi P. Breaking Down SERS Detection Limit: Engineering of a Nanoporous Platform for High Sensing and Technology. Nanomaterials. 2022; 12(10):1737. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12101737
Chicago/Turabian StyleScaglione, Federico, Livio Battezzati, and Paola Rizzi. 2022. "Breaking Down SERS Detection Limit: Engineering of a Nanoporous Platform for High Sensing and Technology" Nanomaterials 12, no. 10: 1737. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12101737