Sea Urchin-like Si@MnO2@rGO as Anodes for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
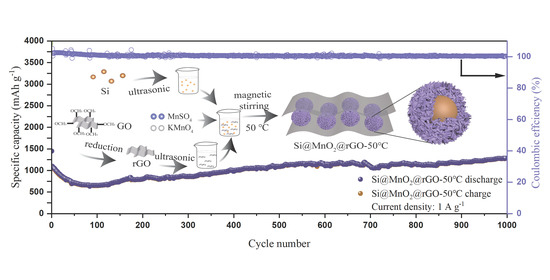
2.2. Preparation of Si@MnO2@rGO
2.3. Electrochemical Measurements
2.4. Materials’ Characterization
3. Results and Discussion
3.1. Anode Material Design and Morphology Characterization
3.2. Lithium-Ion Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mo, R.W.; Li, F.; Tan, X.Y.; Xu, P.C.; Tao, R.; Shen, G.R.; Lu, X.; Liu, F.; Shen, L.; Xu, B.; et al. High-quality mesoporous graphene particles as high-energy and fast-charging anodes for lithium-ion batteries. Nat. Commun. 2019, 10, 1474. [Google Scholar] [CrossRef] [Green Version]
- Grey, P.; Hall, D.S. Prospects for lithium-ion batteries and beyond-a 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; McDowell, M.T.; Jackson, A.; Cha, J.J.; Hong, S.S.; Cui, Y. New Nanostructured Li2S/Silicon Rechargeable Battery with High Specific Energy. Nano Lett. 2010, 10, 1486–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, F.; Li, G.C.; Wang, X.C.; Chen, X.X.; Fu, L.; Liu, X.X.; Mao, E.Y.; Liu, J.; Yang, X.L.; Qian, C.X.; et al. Manipulating Oxidation of Silicon with Fresh Surface Enabling Stable Battery Anode. Nano Lett. 2021, 21, 3127–3133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.R.; Xiang, Y.X.; Xu, L.F.; Luo, H.; Wang, B.L.; Liu, Y. Controlling Surface Oxides in Si/C Nanocomposite Anodes for High-Performance Li-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801718. [Google Scholar] [CrossRef]
- Zheng, T.Y.; Jia, Z.; Lin, N.; Langer, T.; Lux, S.; Lund, I.; Gentschev, A.C.; Qiao, J.; Liu, G. Molecular Spring Enabled High-Performance Anode for Lithium Ion Batteries. Polymers 2017, 9, 657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, H.W.; Li, Q.; Liu, D.Q.; Hou, X.Y.; Bai, S.; Lin, S.M.; He, D.Y. High-yield fabrication of graphene-wrapped silicon nanoparticles for self-support and binder-free anodes of lithium-ion batteries. J. Alloy. Compd. 2018, 744, 243–251. [Google Scholar] [CrossRef]
- Gao, R.S.; Tang, J.; Tang, S.; Zhang, K.; Ozawa, K.; Qin, L.C. Biomineralization-inspired: Rapid preparation of a silicon-based composite as a high-performance lithium-ion battery anode. J. Mater. Chem. A 2021, 9, 11614–11622. [Google Scholar] [CrossRef]
- Fang, R.; Miao, C.; Mou, H.Y.; Xiao, W. Facile synthesis of Si@TiO2@rGO composite with sandwich-like nanostructure as superior performance anodes for lithium ion batteries. J. Alloy. Compd. 2020, 818, 152884. [Google Scholar] [CrossRef]
- Hassan, F.; Batmaz, R.; Li, J.D.; Wang, X.L.; Xiao, X.C.; Yu, A.P.; Chen, Z.W. Evidence of covalent synergy in silicon–sulfur–graphene yielding highly efficient and long-life lithium-ion batteries. Nat. Commun. 2015, 26, 8597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Mao, Z.M.; Lai, L.F.; Okubo, M.; Song, Y.H.; Zhou, Y.J.; Liu, X.; Huang, W. Sub-micron silicon/pyrolyzed carbon@natural graphite self-assembly composite anode material for lithium-ion batteries. Chem. Eng. J. 2017, 313, 187–196. [Google Scholar] [CrossRef]
- Dai, J.Y.; Liao, J.; He, M.Y.; Yang, M.M.; Wu, K.P.; Yao, W.T. Si@SnS2-rGO composite anode material with high lithium-ion battery capacity. ChemSusChem 2019, 12, 5092–5098. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Song, Y.W.; Zhang, C.F.; He, J.J.; Li, X.D.; Wang, X.; Wang, N.; Li, Y.L.; Huang, C.S. Porous 3D Silicon-Diamondyne Blooms Excellent Storage and Diffusion Properties for Li, Na, and K Ions. Adv. Energy Mater. 2021, 11, 2101197. [Google Scholar] [CrossRef]
- Wu, Y.J.; Chen, Y.A.; Huang, C.L.; Su, J.T.; Hsieh, C.T.; Lu, S.Y. Small highly mesoporous silicon nanoparticles for high performance lithium ion based energy storage. Chem. Eng. J. 2020, 400, 125958. [Google Scholar] [CrossRef]
- Liang, B.R.; Zhu, S.S.; Wang, J.C.; Liang, X.Q.; Huang, H.F.; Huang, D.; Zhou, W.Z.; Xu, S.K.; Guo, J. Silicon-doped FeOOH nanorods@graphene sheets as high-capacity and durable anodes for lithium-ion batteries. Appl. Surf. Sci. 2021, 550, 149330. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Mu, Z.J.; Lai, J.P.; Chao, Y.G.; Yang, Y.; Zhou, P.; Li, Y.J.; Yang, W.X.; Xia, Z.H.; Guo, S.J. MXene/Si@SiOX@C Layer-by-Layer Superstructure with Auto-Adjustable Function for Superior Stable Lithium Storage. ACS Nano 2019, 13, 2167–2175. [Google Scholar] [PubMed]
- Suo, G.Q.; Zhang, J.Q.; Li, D.; Yu, Q.Y.; Wang, W.A.; He, M.; Feng, L.; Hou, X.J.; Yang, Y.L.; Ye, X.H.; et al. N-doped carbon/ultrathin 2D metallic cobalt selenide core/sheath flexible framework bridged by chemical bonds for high-performance potassium storage. Chem. Eng. J. 2020, 388, 124396. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, Y.H.; Shi, Q.; Shi, H.W.; Xue, D.F.; Shi, F.N. Highly efficient Co3O4/CeO2 heterostructure as anode for lithium-ion batteries. J. Colloid Interf. Sci. 2020, 585, 705–715. [Google Scholar] [CrossRef]
- Li, D.P.; Dai, L.N.; Ren, X.H.; Ji, F.J.; Sun, Q.; Zhang, Y.M.; Ci, L.J. Foldable Potassium-Ion Batteries Enabled by Free-standing and Flexible SnS2@C Nanofibers. Energy Environ. Sci. 2021, 14, 424–436. [Google Scholar] [CrossRef]
- Pham, T.K.; Shin, J.H.; Karima, N.C.; Jun, Y.S.; Jeong, S.K.; Cho, N.; Lim, S.N. Application of recycled Si from industrial waste towards Si/rGO composite material for long lifetime lithium-ion battery. J. Power Sources 2021, 506, 230244. [Google Scholar] [CrossRef]
- Chen, X.F.; Yang, X.F.; Pan, F.L.; Zhang, T.T.; Zhu, X.Y.; Qiu, J.Y.; Li, M.; Mu, Y.; Ming, H. Fluorine-functionalized core-shell Si@C anode for a high-energy lithium-ion full battery. J. Alloy. Compd. 2021, 884, 160945. [Google Scholar] [CrossRef]
- Tu, W.M.; Bai, Z.Y.; Deng, Z.; Zhang, H.N.; Tang, H.L. In-Situ Synthesized Si@C Materials for the Lithium Ion Battery: A Mini Review. Nanomaterials 2019, 9, 432. [Google Scholar] [CrossRef] [Green Version]
- Gou, W.W.; Kong, X.Z.; Wang, Y.P.; Ai, Y.L.; Liang, S.Q.; Pan, A.Q.; Cao, G.Z. Yolk-shell structured V2O3 microspheres wrapped in N, S co-doped carbon as Pea-Pod nanofibers for high-capacity lithium ion batteries. Chem. Eng. J. 2019, 374, 545–553. [Google Scholar] [CrossRef]
- Wu, F.F.; Gao, X.B.; Xu, X.L.; Jiang, Y.N.; Gao, X.L.; Yin, R.L.; Shi, W.H.; Liu, W.X.; Lu, G.; Cao, X.H. MnO2 nanosheet-assembled hollow polyhedron grown on carbon cloth for flexible aqueous zinc-ion batteries. ChemSusChem 2020, 13, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; He, J.F.; Dai, M.Z.; Zhao, D.P.; Wu, X.; Liu, B.D. Emerging CoMn-LDH@MnO2 electrode materials assembled using nanosheets for flexible and foldable energy storage devices. J. Energy Chem. 2020, 45, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Ren, J.H.; Xu, T.; Feng, A.L.; Hu, K.; Yu, N.F.; Xia, Y.B.; Zhu, Y.S.; Huang, Z.Y.; Wu, G.L. Covalent Bonding of Si Nanoparticles on Graphite Nanosheets as Anodes for Lithium-Ion Batteries Using Diazonium Chemistry. Nanomaterials 2019, 9, 1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.S.; Xiao, Y.Y.; Liu, Y.T.; Han, P.Y.; Qin, G.H. Mesoporous MnO2 based composite electrode for efficient alkali-metal-ion storage. Chem. Eng. J. 2020, 380, 122487. [Google Scholar] [CrossRef]
- Wang, B.Y.; Wei, Y.H.; Fang, H.Y.; Qiu, X.L.; Zhang, Q.B.; Wu, H.; Wang, Q.; Zhang, Y.; Ji, X.B. Mn-substituted tunnel-type polyantimonic acid confined in a multidimensional integrated architecture enabling superfast-charging lithium-ion battery anodes. Adv. Sci. 2021, 8, 2002866. [Google Scholar] [CrossRef]
- Pi, W.B.; Mei, T.; Li, J.; Wang, J.Y.; Li, J.H.; Wang, X.B. Durian-like NiS2@rGO nanocomposites and their enhanced rate performance. Chem. Eng. J. 2018, 335, 275–281. [Google Scholar] [CrossRef]
- Mei, L.; Xu, C.; Yang, T.; Ma, J.M.; Chen, L.B.; Li, Q.H.; Wang, T.H. Superior electrochemical performance of ultrasmall SnS2 nanocrystals decorated on flexible RGO in lithium-ion batteries. J. Mater. Chem. A 2013, 1, 8658–8664. [Google Scholar] [CrossRef]
- Rana, M.; He, Q.; Luo, B.; Lin, T.; Ran, L.B.; Li, M.; Gentle, I.; Knibbe, R. Multifunctional Effects of Sulfonyl-Anchored, Dual-Doped Multilayered Graphene for High Areal Capacity Lithium Sulfur Batteries. ACS Cent. Sci. 2019, 5, 1946–1958. [Google Scholar] [CrossRef]
- Park, S.; Jeong, S.Y.; Lee, T.K.; Park, M.W.; Lim, H.Y.; Sung, J.; Cho, J.; Kwak, S.K.; Hong, S.Y.; Choi, N.S. Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries. Nat. Commun. 2021, 12, 838. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.Y.; Hultman, N.; Cui, D.; McJeon, H.; Yu, S.; Edwards, M.R.; Sen, A.; Song, K.; Bowman, C.; Clarke, L.; et al. A plant-by-plant strategy for high-ambition coal power phaseout in China. Nat. Commun. 2021, 12, 1468. [Google Scholar] [CrossRef]
- Zhao, T.M.; Zheng, C.W.; He, H.X.; Guan, H.Y.; Zhong, T.Y.; Xing, L.L.; Xue, X.Y. A self-powered biosensing electronic-skin for real-time sweat Ca2+ detection and wireless data transmission. Smart Mater. Struct. 2019, 28, 085015. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Xu, X.Y.; Cao, C.B.; Xia, M.; Luo, Y.J. Scalable 2D Mesoporous Silicon Nanosheets for High-Performance Lithium-Ion Battery Anode. Small 2018, 14, 1703361. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.M.; Han, Y.C.; Qin, L.N.; Guan, H.Y.; Xing, L.L.; Li, X.J.; Xue, X.Y.; Li, G.L.; Zhan, Y. Bidirectional modulation of neural plasticity by self-powered neural stimulation. Nano Energy 2021, 85, 106006. [Google Scholar] [CrossRef]
- Liu, H.D.; Hu, Z.L.; Su, Y.Y.; Ruan, H.B.; Hu, R.; Zhang, L. MnO2 nanorods/3D-rGO composite as high performance anode materials for Li-ion batterie. Appl. Surf. Sci. 2017, 392, 777–784. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D.X.; Liu, W.; Shi, B.; Guo, R.; Pei, H.J.; Xie, J.Y. A three-dimensional core-shell nanostructured composite of polypyrrole wrapped MnO2/reduced graphene oxide/carbon nanotube for high performance lithium ion batteries. J. Colloid Interf. Sci. 2017, 493, 241–248. [Google Scholar] [CrossRef]
- Xu, P.; Chen, H.Y.; Zhou, X.; Xiang, H.F. Gel polymer electrolyte based on PVDF-HFP matrix composited with rGO-PEG-NH2 for high-performance lithium ion battery. J. Membr. Sci. 2021, 617, 118660. [Google Scholar] [CrossRef]
- Li, J.B.; Ding, Z.B.; Li, J.L.; Wang, C.Y.; Pan, L.K.; Wang, G.X. Synergistic coupling of NiS1.03 nanoparticle with S-doped reduced graphene oxide for enhanced lithium and sodium storage. Chem. Eng. J. 2020, 407, 127199. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Hu, R.; Liu, B.Y.; Zhang, Y.Y.; Zhu, K.; Yan, J.; Ye, K.; Cheng, K.; Wang, G.L.; Cao, D.X. MXene-derived TiO2/reduced graphene oxide composite with an enhanced capacitive capacity for Li-ion and K-ion batteries. J. Mater. Chem. A 2019, 7, 5363–5372. [Google Scholar] [CrossRef]
- Yao, W.Q.; Wu, S.B.; Zhan, L.; Wang, Y.L. Two-dimensional Porous Carbon-coated Sandwich-like Mesoporous SnO2/Graphene/Mesoporous SnO2 Nanosheets towards High-Rate and Long Cycle Life Lithium-ion Batteries. Chem. Eng. J. 2018, 361, 329–341. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.H.; Kim, N.; Sung, J.; Cho, J. Integration of Graphite and Silicon Anodes for the Commercialization of High-Energy Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 59, 110–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.H.; Zhang, Y.P.; Liu, T.F.; Gao, X.H.; Li, S.Y.; Ling, M.; Liang, C.D.; Zheng, J.C.; Lin, Z. Silicon Anode with High Initial Coulombic Efficiency by Modulated Trifunctional Binder for High-Areal-Capacity Lithium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1903110. [Google Scholar] [CrossRef]
- Keller, C.; Desrues, A.; Karuppiah, S.; Martin, E.; Alper, J.; Boismain, F.; Villevieille, C.; Herlin-Boime, N.; Haon, C.; Chenevier, P. Effect of Size and Shape on Electrochemical Performance of Nano-Silicon-Based Lithium Battery. Nanomaterials 2021, 11, 307. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Cai, X.; Yu, X.Y.; Wang, H.Q.; Li, Q.Y.; Fang, Y.P. Zinc-assisted Mechanochemical Coating of Reduced Graphene Oxide Thin Layer on Silicon Microparticles for Efficient Lithium-ion Battery Anodes. Sustain. Energy Fuels. 2019, 3, 1258–1268. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.S.; Wang, M.S.; Wang, G.L.; Ma, Y.; Huang, Y.; Zheng, J.M. Dual Carbonaceous Materials Synergetic Protection Silicon as a High-Performance Free-Standing Anode for Lithium-Ion Battery. Nanomaterials 2019, 9, 650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laruelle, S.; Grugeon, S.; Poizot, P.; Dolle, M.; Dupont, L.; Tarascon, J.M. On the Origin of the Extra Electrochemical Capacity Displayed by MO/Li Cells at Low Potential. J. Electrochem. Soc. 2002, 149, A627–A634. [Google Scholar] [CrossRef]
- Hao, Q.; Wang, J.P.; Xu, C.X. Facile preparation of Mn3O4 octahedra and their long-term cycle life as an anode material for Li-ion batteries. J. Mater. Chem. A 2014, 2, 87–93. [Google Scholar] [CrossRef]
- Li, L.; Guo, Z.P.; Du, A.J.; Liu, H.K. Rapid microwave-assisted synthesis of Mn3O4-graphene nanocomposite and its lithium storage properties. J. Mater. Chem. 2012, 22, 3600–3605. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Jiang, Z.J.; Chen, B.H.; Jiang, Z.Q.; Cheng, S.; Rong, H.B.; Huang, J.L.; Liu, M.L. Morphology and crystal phase evolution induced performance enhancement of MnO2 grown on reduced graphene oxide for lithium ion batteries. J. Mater. Chem. A 2016, 4, 2643–2650. [Google Scholar] [CrossRef]
- Kim, K.; Daniel, G.; Kessler, V.G.; Seisenbaeva, G.A.; Pol, V.G. Basic Medium Heterogeneous Solution Synthesis of alpha-MnO2 Nanoflakes as an Anode or Cathode in Half Cell Configuration (vs. Lithium) of Li-Ion Batteries. Nanomaterials 2018, 8, 608. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wu, X.; Wang, B. Performance modulation of energy storage devices: A case of Ni-Co-S electrode materials. Chem. Eng. J. 2020, 392, 123651. [Google Scholar] [CrossRef]
- Wu, X.; Yao, S.Y. Flexible electrode materials based on WO3 nanotube bundles for high performance energy storage devices. Nano Energy 2017, 42, 143–150. [Google Scholar] [CrossRef]
- Zhang, D.A.; Wang, Q.; Wang, Q.; Sun, J.; Xing, L.L.; Xue, X.Y. High capacity and cyclability of hierarchical MoS2/SnO2 nanocomposites as the cathode of lithium-sulfur battery. Electrochim. Acta. 2015, 173, 472–482. [Google Scholar] [CrossRef]
- Chen, Y.X.; Yan, Y.J.; Liu, X.L.; Zhao, Y.; Wu, X.Y.; Zhou, J.; Wang, Z.F. Porous Si/Fe2O3 Dual Network Anode for Lithium–Ion Battery Application. Nanomaterials 2020, 10, 2331. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, K.; Zhou, Y.L.; Xia, Y.B.; Yu, N.F.; Wu, G.L.; Zhu, Y.S.; Wu, Y.P.; Huang, H.B. A Facile, One-Step Synthesis of Silicon/Silicon Carbide/Carbon Nanotube Nanocomposite as a Cycling-Stable Anode for Lithium Ion Batteries. Nanomaterials 2019, 9, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, N.; Li, J.J.; Wang, X.C.; Zhang, C.L.; Liu, G.; Li, X.H.; Qu, J.; Peng, Z.; Zhu, X.Y.; Zhang, L. Flexible Carbon Nanotubes Confined Yolk-Shelled Silicon-Based Anode with Superior Conductivity for Lithium Storage. Nanomaterials 2021, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Hwang, J.Y.; Shin, H.J.; Jeong, M.G.; Chung, K.Y.; Sun, Y.K.; Jung, H.G. Nano/Microstructured Silicon–Carbon Hybrid Composite Particles Fabricated with Corn Starch Biowaste as Anode Materials for Li-Ion Batteries. Nano Lett. 2019, 20, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Argyropoulos, D.P.; Zardalidis, G.; Giotakos, P.; Daletou, M.; Farmakis, F. Study of the Role of Void and Residual Silicon Dioxide on the Electrochemical Performance of Silicon Nanoparticles Encapsulated by Graphene. Nano Lett. 2021, 11, 2864. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.F.; Li, J.Y.; Xie, Y.Y.; Guan, D.S.; Yuan, C. A Multilayered Silicon-Reduced Graphene Oxide Electrode for High Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces. 2015, 7, 7855–7862. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Ji, X.; Wu, F.Z.; Yang, W.L.; Dai, X.Y.; Xu, X.J.; Wang, J.; Guo, D.; Chen, M.L. Facile fabrication of a three-dimensional coral-like silicon nanostructure coated with a C/rGO double layer by using the magnesiothermic reduction of silica nanotubes for high-performance lithium-ion battery anodes. J. Alloys Compd. 2021, 863, 158569. [Google Scholar] [CrossRef]
- Wang, D.X.; Wang, Y.; Li, Q.Y.; Guo, W.B.; Zhang, F.C.; Niu, S.S. Urchin-like alpha-Fe2O3/MnO2 hierarchical hollow composite microspheres as lithium-ion battery anodes. J. Power Sources. 2018, 393, 186–192. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhao, T.B. Reduced graphene oxide anchored with MnO2 nanorods as anode for high rate and long cycle Lithium ion batteries. Electrochim. Acta. 2016, 201, 165–171. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, M.; Wang, Q.; Zhao, X.; Song, Y.; Zhao, T.; Sun, J. Sea Urchin-like Si@MnO2@rGO as Anodes for High-Performance Lithium-Ion Batteries. Nanomaterials 2022, 12, 285. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020285
Liu J, Wang M, Wang Q, Zhao X, Song Y, Zhao T, Sun J. Sea Urchin-like Si@MnO2@rGO as Anodes for High-Performance Lithium-Ion Batteries. Nanomaterials. 2022; 12(2):285. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020285
Chicago/Turabian StyleLiu, Jiajun, Meng Wang, Qi Wang, Xishan Zhao, Yutong Song, Tianming Zhao, and Jing Sun. 2022. "Sea Urchin-like Si@MnO2@rGO as Anodes for High-Performance Lithium-Ion Batteries" Nanomaterials 12, no. 2: 285. https://0-doi-org.brum.beds.ac.uk/10.3390/nano12020285