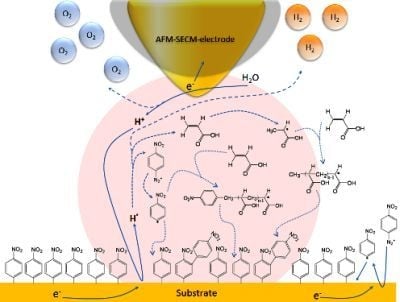
Nano-Electrochemistry and Nano-Electrografting with an Original Combined AFM-SECM
Abstract
:1. Introduction

2. Experimental Section
2.1. Hydrogel-Cell Preparation
2.2. Sharp AFM-SECM-Electrode Fabrication and Characterizations
2.2.1. Tip Etching
2.2.2. Tip Insulation
2.2.3. Selective Exposure of the Sharp Apex of the Microwire


2.2.4. AFM-SECM-Electrode Electrical Connection
2.2.5. AFM-SECM-Electrode Normal Spring Constant Calculation
2.3. AFM-SECM Experiments Details
2.3.1. AFM-SECM/Agarose Electrochemical System Validation Experiment
2.3.2. Nano-Electrografting Experiment

3. Results and Discussion





4. Conclusions
References
- Bard, A.J.; Mirkin, M.V. Scanning Electrochemical Microscopy; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Mirkin, M.V.; Horrocks, B.R. Electroanalytical measurements using the scanning electrochemical microscope. Anal. Chim. Acta 2000, 406, 119–146. [Google Scholar] [CrossRef]
- Jones, C.E.; Macpherson, J.V.; Barber, Z.H.; Somekh, R.E.; Unwin, P.R. Simultaneous topographical and amperometric imaging of surfaces in air: Towards a combined scanning force-scanning electrochemical microscope (SF-SECM). Electrochem. Commun. 1999, 1, 55–60. [Google Scholar]
- Kranz, C.; Kueng, A.; Lugstein, A.; Bertagnolli, E.; Mizaikoff, B. Mapping of enzyme activity by detection of enzymatic products during AFM imaging with integrated SECM-AFM probes. Ultramicroscopy 2004, 100, 127–134. [Google Scholar] [CrossRef]
- Macpherson, J.V.; Unwin, P.R. Noncontact electrochemical imaging with combined scanning electrochemical atomic force microscopy. Anal. Chem. 2001, 73, 550–557. [Google Scholar] [CrossRef]
- Gardner, C.E.; Macpherson, J.V. Peer reviewed: Atomic force microscopy probes go electrochemical. Anal. Chem. 2002, 74, 576A–584A. [Google Scholar]
- Lugstein, A.; Bertagnolli, E.; Kranz, C.; Kueng, A.; Mizaikoff, B. Integrating micro- and nano-electrodes into atomic force microscopy cantilevers using focused ion beam techniques. Appl. Phys. Lett. 2002, 81, 349–351. [Google Scholar] [CrossRef]
- Kranz, C.; Friedbacher, G.; Mizaikoff, B.; Lugstein, A.; Bertagnolli, E. Integrating an ultramicroelectrode in an AFM cantilever: Combined technology for enhanced information. Anal. Chem. 2001, 73, 2491–2500. [Google Scholar] [CrossRef]
- Macpherson, J.V.; Unwin, P.R. Combined scanning electrochemical-atomic force microscopy. Anal. Chem. 2000, 72, 276–285. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical biosensors—Sensor principles and architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [Green Version]
- Lugstein, A.; Bertagnolli, E.; Kranz, C.; Mizaikoff, B. Fabrication of a ring nanoelectrode in an AFM tip: Novel approach towards simultaneous electrochemical and topographical imaging. Surf. Interface Anal. 2002, 33, 146–150. [Google Scholar] [CrossRef]
- Sekine, S.; Kaji, H.; Nishizawa, M. Integration of an electrochemical-based biolithography technique into an AFM system. Anal. Bioanal. Chem. 2008, 391, 2711–2716. [Google Scholar] [CrossRef]
- Kueng, A.; Kranz, C.; Lugstein, A.; Bertagnolli, E.; Mizaikoff, B. Integrated AFM-SECM in tapping mode: Simultaneous topographical and electrochemical imaging of enzyme activity. Angew. Chem. Int. Ed. 2003, 42, 3238–3240. [Google Scholar] [CrossRef]
- Ghorbal, A.; Grisotto, F.; Charlier, J.; Palacin, S.; Goyer, C.; Demaille, C. Localized electrografting of vinylic monomers on a conducting substrate by means of an integrated electrochemical AFM probe. Chem. Phys. Chem. 2009, 10, 1053–1057. [Google Scholar] [CrossRef]
- Ghorbal, A.; Grisotto, F.; Laudé, M.; Charlier, J.; Palacin, S. The in situ characterization and structuring of electrografted polyphenylene films on silicon surfaces. An AFM and XPS study. J. Colloid. Interface Sci. 2008, 328, 308–313. [Google Scholar] [CrossRef]
- Burrell, M.C.; Codela, P.J.; Fontana, J.A.; Chera, J.J.; McConnell, M.D. Interfacial reactions at copper surfaces coated with polyimide films prepared from poly(amide-acid) precursors. J. Vac. Sci. Technol. A 1989, 7, 55–58. [Google Scholar]
- Viel, P.; Dubois, L.; Lyskawa, J.; Sallé, M.; Palacin, S. New concept to remove heavy metals from liquid waste based on electrochemical pH-switchable immobilized ligands. Appl. Surf. Sci. 2007, 253, 3263–3269. [Google Scholar] [CrossRef]
- Palacin, S.; Bureau, C.; Charlier, J.; Deniau, G.; Mouanda, B.; Viel, P. Molecule-to-metal bonds: Electrografting polymers on conducting surfaces. Chem. Phys. Chem. 2004, 10, 1468–1481. [Google Scholar]
- Tourillon, G.; Garrett, R.; Lazarz, N.; Raynaud, M.; Reynaud, C.; Lecayon, G.; Viel, P. A NEXAFS study of thin polyacrylonitrile films electrochemically deposited on Ni: The effect of the film thickness and annealing treatment. J. Electrochem. Soc. 1990, 137, 2499–2501. [Google Scholar] [CrossRef]
- Deniau, G.; Azoulay, L.; Jegou, P.; le Chevallier, G.; Palacin, S. Carbon-to-metal bonds: Electrochemical reduction of 2-butenenitrile. Surf. Sci. 2006, 600, 675–684. [Google Scholar] [CrossRef]
- Zhang, X.; Bell, J.P. Studies of arenediazonium salts as a new class of electropolymerization initiator. J. App. Polym. Sci. 1999, 73, 2265–2272. [Google Scholar] [CrossRef]
- Deniau, G.; Azoulay, L.; Bourgerolles, L.; Palacin, S. Surface electroinitiated emulsion polymerization: Grafted organic coatings from aqueous solutions. Chem. Mater. 2006, 18, 5421–5428. [Google Scholar] [CrossRef]
- Hazra, A.; Sen, P.; Basumallick, I.N. Modified hydrogel—An unique material for electrochemical studies. J. New Mater. Electrochem. Syst. 2001, 4, 89–92. [Google Scholar]
- Kaneko, M.; Mochizuki, N.; Suzuki, K.; Shiroishi, H.; Ishikawa, K. Molecular reactor for solution chemistry. Chem. Lett. 2002, 31, 530–531. [Google Scholar]
- Suzuki, K.; Shiroishi, H.; Hoshino, M.; Kaneko, M. New quasi-solid materials as a medium for photochemical reactions. J. Phys. Chem. A 2003, 107, 5523–5527. [Google Scholar] [CrossRef]
- Mouanda, B.; Eyeffa, V.; Palacin, S. Agarose-based hydrogel as an electrografting cell. J Appl Electrochem. 2009, 39, 313–320. [Google Scholar] [CrossRef]
- Demaille, C. Gold Spherical Microelectrodes. In Handbook of Electrochemistry, 1st; Cynthia, G.Z., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Chapter 6; pp. 226–235. [Google Scholar]
- Abbou, J.; Demaille, C.; Dret, M.; Moiroux, J. Fabrication of submicrometer-sized gold electrodes of controlled geometry for scanning electrochemical-atomic force microscopy. Anal. Chem. 2002, 74, 6355–6363. [Google Scholar] [CrossRef]
- Sader, J.E. Susceptibility of atomic force microscope cantilevers to lateral forces. Rev. Sci. Instrum. 2003, 74, 2438–2443. [Google Scholar] [CrossRef]
- Haugstad, G.; Gladfelter, W.L. Force-displacement measurements in a beam-reflection scanning force microscope: Calibration issues. Ultramicroscopy 1994, 54, 31–40. [Google Scholar] [CrossRef]
- Kueng, A.; Kranz, C.; Mizaikoff, B.; Lugstein, A.; Bertagnolli, E. Combined scanning electrochemical atomic force microscopy for tapping mode imaging. Appl. Phys. Lett. 2003, 82, 1592–1594. [Google Scholar] [CrossRef]
- Rodríguez-López, J.; Ritzert, N.L.; Mann, J.A.; Tan, C.; Dichtel, W.R.; Abruña, H.D. Quantificationof the surface diffusion of tripodal binding motifs on graphene using scanning electrochemical microscopy. J. Am. Chem. Soc. 2012, 134, 6224–6236. [Google Scholar]
- Grisotto, F.; Ghorbal, A.; Goyer, C.; Charlier, J.; Palacin, S. Direct SECM localized electrografting of vinylic monomers on conducting substrate. Chem. Mater. 2011, 23, 1396–1405. [Google Scholar] [CrossRef]
- Charlier, J.; Grisotto, F.; Ghorbal, A.; Goyer, C.; Palacin, S. Localized electrografting of diazonium salts in the SECM environment. Mater. Sci. Forum 2013, 730–732, 221–226. [Google Scholar]
- Borgwarth, K.; Heinze, J. Increasing the resolution of the scanning electrochemical microscope using a chemical lens: Application to silver deposition. J. Electrochem. Soc. 1999, 146, 3285–3289. [Google Scholar] [CrossRef]
- Tessier, L.; Chancolon, J.; Alet, P.-J.; Trenggono, A.; Mayne-L’Hermite, M.; Deniau, G.; Jegou, P.; Palacin, S. Grafting organic polymer films on surfaces of carbon nanotubes by surface electroinitiated emulsion polymerization. Phys. Status Solidi A 2008, 205, 1412–1418. [Google Scholar] [CrossRef]
- Xie, Y.; Osteryoung, J.G. The coupling of diffusion, migration and chemical equilibrium during the voltammetric reduction of weak polyprotic acids. J. Electroanal. Chem. 1997, 439, 163–171. [Google Scholar] [CrossRef]
- Ciszkowska, M.; Stojek, Z.; Morris, S.E.; Osteryoung, J.G. Steady-state voltammetry of strong and weak acids with and without supporting electrolyte. Anal. Chem. 1992, 64, 2372–2377. [Google Scholar] [CrossRef]
- Piner, R.D.; Zhu, J.; Xu, F.; Hong, S.; Mirkin, C.A. “Dip-Pen” nanolithography. Science 1999, 283, 661–663. [Google Scholar] [CrossRef]
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ghorbal, A.; Grisotto, F.; Charlier, J.; Palacin, S.; Goyer, C.; Demaille, C.; Brahim, A.B. Nano-Electrochemistry and Nano-Electrografting with an Original Combined AFM-SECM. Nanomaterials 2013, 3, 303-316. https://0-doi-org.brum.beds.ac.uk/10.3390/nano3020303
Ghorbal A, Grisotto F, Charlier J, Palacin S, Goyer C, Demaille C, Brahim AB. Nano-Electrochemistry and Nano-Electrografting with an Original Combined AFM-SECM. Nanomaterials. 2013; 3(2):303-316. https://0-doi-org.brum.beds.ac.uk/10.3390/nano3020303
Chicago/Turabian StyleGhorbal, Achraf, Federico Grisotto, Julienne Charlier, Serge Palacin, Cédric Goyer, Christophe Demaille, and Ammar Ben Brahim. 2013. "Nano-Electrochemistry and Nano-Electrografting with an Original Combined AFM-SECM" Nanomaterials 3, no. 2: 303-316. https://0-doi-org.brum.beds.ac.uk/10.3390/nano3020303