Hybrid Mesoporous Silicas and Microporous POSS-Based Frameworks Incorporating Evaporation-Induced Self-Assembly
Abstract
:1. Introduction

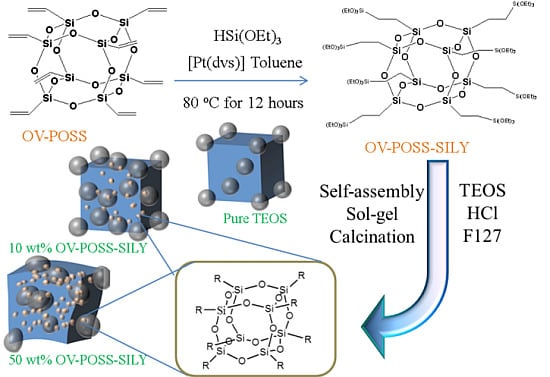
2. Results and Discussion




| OV-POSS-SILY | d (nm) | Pore Size (nm) | SBET (m2/g) | SM (m2/g) | Pore Volume (cm3/g) | Micropore Volume (cm3/g) |
|---|---|---|---|---|---|---|
| 0% | 10.8 | 4.8 | 831 | 185 | 0.71 | 0.076 |
| 10% | 9.97 | 4.0 | 809 | 264 | 0.49 | 0.114 |
| 20% | 9.35 | 3.4 | 622 | 272 | 0.34 | 0.121 |
| 30% | 9.35 | 2.5 | 418 | 243 | 0.21 | 0.112 |
| 50% | 8.66 | - | 537 | 313 | 0.32 | 0.145 |
| 100% | - | - | 519 | 252 | 0.26 | 0.115 |



3. Experimental Section
3.1. Materials
3.2. OV-POSS-SILY
3.3. Mesoporous Silicas Containing POSS
3.4. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.; Ishida, Y.; Maeda, R.; Tokita, M.; Horiuchi, S.; Hayakawa, T. Alkylated cage silsesquioxane forming a long-range straight ordered hierarchical lamellar nanostructure. Langmuir 2014, 30, 9797–9803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ye, Q.; Neo, W.T.; Song, J.; Yan, H.; Zong, Y.; Tang, B.Z.; Hor, T.S.A.; Xu, J.W. Electrospun aggregation-induced emission active POSS-based porous copolymer films for detection of explosives. Chem. Commun. 2014, 50, 13785–13788. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, Z.; Ma, L.; Chen, G.; Li, Q. Hierarchical assembly of amphiphilic POSS-cyclodextrin molecules and azobenzene end-capped polymers. Macromolecules 2014, 47, 5739–5748. [Google Scholar] [CrossRef]
- Hu, M.B.; Hou, Z.Y.; Hao, W.Q.; Xiao, Y.; Yu, W.; Ma, C.; Ren, L.J.; Zheng, P.; Wang, W. POM-Organic-POSS Cocluster: Creating A dumbbell-shaped hybrid molecule for programming hierarchical supramolecular nanostructures. Langmuir 2013, 29, 5714–5722. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Chujo, Y. Advanced functional materials based on polyhedral oligomeric silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 1733–1746. [Google Scholar] [CrossRef]
- Lin, Y.C.; Kuo, S.W. Hierarchical self-assembly structures of POSS-containing polypeptide block copolymers synthesized using a combination of ATRP, ROP and click chemistry. Polym. Chem. 2012, 3, 882–891. [Google Scholar] [CrossRef]
- Li, J.G.; Chung, C.Y.; Kuo, S.W. Transformations and enhanced long-range ordering of mesoporous phenolic resin templated by poly(ethylene oxide-b-ε-caprolactone) block copolymers blended with star poly(ethylene oxide)-functionalized silsesquioxane (POSS). J. Mater. Chem. 2012, 22, 18583–18595. [Google Scholar] [CrossRef]
- Jiang, B.; Tao, W.; Lu, X.; Liu, Y.; Jin, H.; Pang, Y.; Sun, X.; Yan, D.; Zhou, Y. A POSS-based supramolecular amphiphile and its hierarchical self-assembly behaviors. Macromol. Rapid Commun. 2012, 33, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Laine, R.M.; Roll, M.F. Polyhedral phenylsilsesquioxanes. Macromolecules 2011, 44, 1073–1109. [Google Scholar] [CrossRef]
- Han, J.; Zheng, Y.C.; Zheng, S.; Li, S.P.; Hu, T.N.; Tang, A.J.; Gao, C. Water soluble octa-functionalized POSS: All-click chemistry synthesis and efficient host–guest encapsulation. Chem. Commun. 2014, 50, 8712–8714. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Chujo, Y. Unique properties of amphiphilic POSS and their applications. Polym. J. 2013, 45, 247–254. [Google Scholar] [CrossRef]
- Yu, X.; Yue, K.; Hsieh, I.F.; Li, Y.; Dong, X.H.; Xin, Y.; Wang, H.F.; Shi, A.C.; Newkome, G.R.; Ho, R.M.; et al. Giant surfactants provide a versatile platform for sub-10-nm nanostructure engineering. Proc. Natl. Acad. Sci. USA 2013, 110, 10078–10083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Yu, X.; Wang, C.L.; Sun, H.J.; Hsieh, I.F.; Li, Y.; Dong, X.H.; Yue, K.; Horn, R.V.; Cheng, S.Z.D. Molecular nanoparticles are unique elements for macromolecular science: From “nanoatoms” to giant molecules. Macromolecules 2014, 47, 1221–1239. [Google Scholar] [CrossRef]
- Moitra, N.; Kanamori, K.; Shimada, T.; Takeda, K.; Ikuhara, Y.H.; Gao, X.; Nakanishi, K. Synthesis of hierarchically porous hydrogen silsesquioxane monoliths and embedding of metal nanoparticles by on-site reduction. Adv. Funct. Mater. 2013, 23, 2714–2722. [Google Scholar] [CrossRef]
- Gandhi, S.; Kumar, P.; Thandavan, K.; Jang, K.; Shin, D.-S.; Vinu, A. Synthesis of a novel hierarchical mesoporous organic–inorganic nanohybrid using polyhedral oligomericsilsesquioxane bricks. New J. Chem. 2014, 38, 2766–2769. [Google Scholar] [CrossRef]
- Li, J.; Liang, G.; Zhu, X.; Yang, S. Exploiting nanoroughness on holographically patterned three-dimensional photonic crystals. Adv. Funct. Mater. 2012, 22, 2980–2986. [Google Scholar] [CrossRef]
- Suzuki, N.; Kiba, S.; Yamauchi, Y. Low dielectric property of novel mesoporous silica/polymer composites using smart molecular caps: Theoretical calculation of air space encapsulated inside mesopores. Microporous Mesoporous Mater. 2010, 138, 123–131. [Google Scholar] [CrossRef]
- Kiba, S.; Okawauchi, Y.; Yanagihara, T.; Murakami, M.; Shimizu, T.; Yamauchi, Y. Mesoporous silica/polymer composites utilizing intelligent caps onto msopore walls toward practical low-dielectric materials. Chem. Asian J. 2009, 4, 1798–1801. [Google Scholar] [PubMed]
- Zhang, L.; Abbenhuis, H.C.L.; Yang, Q.; Wang, Y.-M.; Magusin, P.C.M.M.; Mezari, B.; van Santen, R.A.; Li, C. Mesoporous organic–inorganic hybrid materials built using polyhedral oligomeric silsesquioxane blocks. Angew. Chem. Int. Ed. 2007, 46, 5003–5006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, Q.; Yang, H.; Liu, J.; Xin, H.; Mezari, B.; Magusin, P.C.M.M.; Abbenhuis, H.C.L.; van Santen, R.A.; Li, C. Super-microporous organosilicas synthesized from well-defined nanobuilding units. J. Mater. Chem. 2008, 18, 450–457. [Google Scholar] [CrossRef]
- Seino, M.; Wang, W.; Lofgreen, J.E.; Puzzo, D.P.; Manabe, T.; Ozin, G.A. Low-k periodic mesoporous organosilica with air walls: POSS-PMO. J. Am. Chem. Soc. 2011, 133, 18082–18085. [Google Scholar] [CrossRef] [PubMed]
- Ortel, E.; Fischer, A.; Chuenchom, L.; Polte, J.; Emmerling, F.; Smarsly, B.; Kraehnert, R. New triblock copolymer templates, PEO-PB-PEO, for the synthesis of Titania films with controlled mesopore size, wall thickness, and bimodal porosity. Small 2012, 8, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; Koehn, R.; Doeblinger, M.; Keilbach, A.; Amenitsch, H.; Bein, T. In situ SAXS study on a new mechanism for mesostructure formation of ordered mesoporous carbons: Thermally induced self-assembly. J. Am. Chem. Soc. 2012, 134, 11136–11145. [Google Scholar] [CrossRef] [PubMed]
- Florent, M.; Xue, C.; Zhao, D.; Goldfarb, D. Formation mechanism of cubic mesoporous carbon monolith synthesized by evaporation-induced self-assembly. Chem. Mater. 2012, 24, 383–392. [Google Scholar] [CrossRef]
- Liu, C.C.; Chu, W.C.; Li, J.G.; Kuo, S.W. Mediated competitive hydrogen bonding form mesoporous phenolic resins templated by Poly(ethylene oxide-b-ε-caprolactone-b-l-lactide) triblock copolymers. Macromolecules 2014, 47, 6389–6400. [Google Scholar] [CrossRef]
- Li, J.G.; Chu, W.C.; Jeng, U.S.; Kuo, S.W. In situ monitoring of the reaction-induced self-assembly of phenolic resin templated by diblock copolymers. Macromol. Chem. Phys. 2013, 214, 2115–2123. [Google Scholar] [CrossRef]
- Liu, C.C.; Li, J.G.; Kuo, S.W. Co-template method provides hierarchical mesoporous silicas with exceptionally ultra-low refractive indices. RSC Adv. 2014, 4, 20262–20272. [Google Scholar] [CrossRef]
- Li, J.G.; Chang, Y.H.; Lin, Y.S.; Kuo, S.W. Templating amphiphilic poly(ethylene oxide-b-caprolactone) diblock copolymers provides ordered mesoporous silicas with large tunable pores. RSC Adv. 2012, 2, 12973–12982. [Google Scholar] [CrossRef]
- Li, J.G.; Kuo, S.W. Phase behavior of mesoporous nanostructures templated by amphiphilic crystalline–crystalline diblock copolymers of poly(ethylene oxide-b-ε-caprolactone). RSC Adv. 2011, 1, 1822–1833. [Google Scholar] [CrossRef]
- Li, J.G.; Chu, W.C.; Tu, C.W.; Kuo, S.W. Tunable mesoporous lamellar silicas prepared using poly(ethylene oxide-b-l-lactide) and poly(ethylene-b-ethylene oxide-b-l-lactide) block copolymers as templates. J. Nanosci. Nanotechnol. 2013, 13, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Chen, W.C.; Kuo, S.W. Phase behavior of mesoporous silicas templated by the amphiphilic diblock copolymer poly(ethylene-b-ethylene oxide). Microporous Mesoporous Mater. 2012, 163, 34–41. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, R.B.; Kuo, S.W. Phase behavior of hierarchical mesoporous silicas prepared using ABC triblock copolymers as single templates. RSC Adv. 2013, 3, 17411–17423. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, R.B.; Kuo, S.W. Hierarchical mesoporous silica fabricated from an ABC triblock terpolymer as a single template. Macromol. Rapid Commun. 2012, 33, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.C.; Chiang, S.F.; Li, J.G.; Kuo, S.W. Mesoporous silicas templated by symmetrical multiblock copolymers through evaporationinduced self-assembly. RSC Adv. 2014, 4, 784–793. [Google Scholar] [CrossRef]
- Li, J.G.; Lin, Y.D.; Kuo, S.W. From microphase separation to self-organized mesoporous phenolic resin through competitive hydrogen bonding with double-crystalline diblock copolymers of poly(ethylene oxide-b-ε-caprolactone). Macromolecules 2011, 44, 9295–9309. [Google Scholar] [CrossRef]
- Wan, Y.; Shi, Y.; Zhao, D. Supramolecular aggregates as templates: Ordered mesoporous polymers and carbons. Chem. Mater. 2008, 20, 932–945. [Google Scholar] [CrossRef]
- Valkama, S.; Nykanen, A.; Kosonen, H.; Ramani, R.; Tuomisto, F.; Engelhardt, P.; ten Brinke, G.; Ikkala, O.; Ruokolainen, J. Hierarchical porosity in self-assembled polymers: Post-modification of block copolymer-phenolic resin complexes by pyrolysis allows the control of micro- and mesoporosity. Adv. Funct. Mater. 2007, 17, 183–190. [Google Scholar] [CrossRef]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Cheng, L.; Feng, D.; Wu, Z.; Chen, Z.; Wan, Y.; Stein, A.; Zhao, D. A family of highly ordered mesoporous polymer resin and carbon structures from organic-organic self-assembly. Chem. Mater. 2006, 18, 4447–4464. [Google Scholar] [CrossRef]
- Liu, R.; Shi, Y.; Wan, Y.; Meng, Y.; Zhang, F.; Gu, D.; Chen, Z.; Tu, B.; Zhao, D. Triconstituent co-assembly to ordered mesostructured polymer-silica and carbon-silica nanocomposites and large-pore mesoporous carbons with high surface areas. J. Am. Chem. Soc. 2006, 128, 11652–11662. [Google Scholar] [CrossRef] [PubMed]
- Kosonen, H.; Valkama, S.; Nykanen, A.; Toivanen, M.; ten Brinke, G.; Ruokolainen, J.; Ikkala, O. Functional porous structures based onthe pyrolysis of cured templates of block copolymer and phenolic resin. Adv. Mater. 2006, 18, 201–205. [Google Scholar] [CrossRef]
- Chu, W.C.; Li, J.G.; Kuo, S.W. From flexible to mesoporous polybenzoxazine resins templated by poly(ethylene oxide-b-ε-caprolactone) copolymer through reaction induced microphase separation mechanism. RSC Adv. 2013, 3, 6485–6498. [Google Scholar] [CrossRef]
- Li, J.G.; Tsai, C.-Y.; Kuo, S.W. Fabrication and characterization of inorganic silver and palladium nanostructures within hexagonal cylindrical channels of mesoporous carbon. Polymers 2014, 6, 1794–1809. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Phenolic resin-based carbons with ultra-large mesopores prepared in the presence of poly(ethylene oxide)-poly(butylene oxide)-poly(ethylene oxide) triblock copolymer and trimethyl benzene. Carbon 2013, 51, 45–51. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, J.; Cai, H.; Zhai, Y.; Feng, D.; Deng, Y.; Tu, B.; Zhao, D. A curing agent method to synthesize ordered mesoporous carbons from linear novolac phenolic resin polymers. J. Mater. Chem. 2009, 19, 6536–6541. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, C.; Gu, D.; Yu, T.; Tu, B.; Zhao, D. Thick wall mesoporous carbons with a large pore structure templated from a weakly hydrophobic PEO-PMMA diblock copolymer. J. Mater. Chem. 2008, 18, 91–97. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-G.; Chu, W.-C.; Kuo, S.-W. Hybrid Mesoporous Silicas and Microporous POSS-Based Frameworks Incorporating Evaporation-Induced Self-Assembly. Nanomaterials 2015, 5, 1087-1101. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5021087
Li J-G, Chu W-C, Kuo S-W. Hybrid Mesoporous Silicas and Microporous POSS-Based Frameworks Incorporating Evaporation-Induced Self-Assembly. Nanomaterials. 2015; 5(2):1087-1101. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5021087
Chicago/Turabian StyleLi, Jheng-Guang, Wei-Cheng Chu, and Shiao-Wei Kuo. 2015. "Hybrid Mesoporous Silicas and Microporous POSS-Based Frameworks Incorporating Evaporation-Induced Self-Assembly" Nanomaterials 5, no. 2: 1087-1101. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5021087