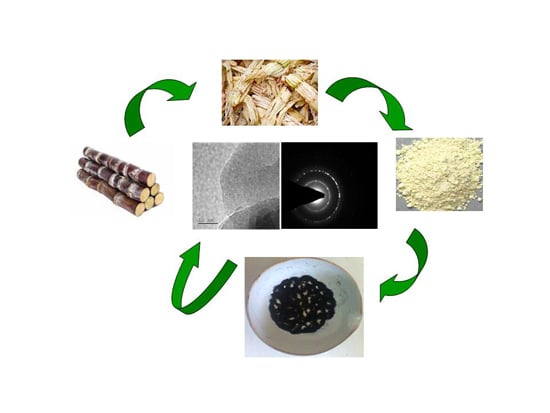
Graphene Oxide Synthesis from Agro Waste
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of GO from Agro Waste

2.2. Characterization of GO
3. Results and Discussion
3.1. X-Ray Diffraction Measurements

3.2. FT-IR Spectroscopy

3.3. SEM and HRTEM Analysis


3.4. Raman Spectroscopy

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chae, H.K.; Siberio-Perez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.; Geim, A.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.; Grigorieva, I.; Firsov, A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Schwierz, F. Graphene transistors. Nat. Nanotechnol. 2010, 5, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhang, L.; He, C.; Qu, Y.; Tian, Y.F.; Lv, Y. Graphene sheets decorated with SnO2 nanoparticles: In situ synthesis and highly efficient materials for cataluminescence gas sensors. J. Mater. Chem. 2011, 21, 5972–5977. [Google Scholar] [CrossRef]
- Si, Y.C.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Lu, G.; Park, S.; Yu, K.; Ruoff, R.S.; Ocola, L.E.; Rosenmann, D.; Chen, J. Toward practical gas sensing with highly reduced graphene oxide: A new signal processing method to circumvent run-to-run and device-to-device variations. ACS Nano 2011, 5, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer grapheme. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for grapheme. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef] [PubMed]
- Eigler, S.; Hu, Y.; Ishii, Y.; Hirsch, A. Controlled functionalization of graphene oxide with sodium azide. Nanoscale 2013, 5, 12136–12139. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. (In German) [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Rider, A.E.; Kumar, S.; Randeniya, L.K.; Ostrikov, K. Vertical graphene gas- and bio-sensors via catalyst-free, reactive plasma reforming of natural honey. Carbon 2013, 60, 221–228. [Google Scholar] [CrossRef]
- Seo, D.H.; Han, Z.J.; Kumar, S.; Ostrikov, K. Structure-controlled, vertical grapheme-based, binder-free electrodes from plasma-reformed butter enhance super capacitor performance. Adv. Energy Mater. 2013, 10, 1316–1323. [Google Scholar] [CrossRef]
- Chandel, A.K.; Antunes, F.A.F.; Anjos, V.; Bell, M.J.V.; Rodrigues, L.N.; Polikarpov, I.; de Azevedo, E.R.; Bernardinelli, O.D.; Rosa, C.A.; Pagnocca, F.C.; et al. Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid-base pretreatment and ethanol production by Scheffersomycesshehatae and Saccharomyces cerevisiae. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef]
- Rezende, C.A.; de Lima, M.A.; Maziero, P.; de Azevedo, E.R.; Garcia, W.; Polikarpov, I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 11, 4–54. [Google Scholar]
- Velmurugan, R.; Muthukumar, K. Utilization of sugarcane bagasse for bioethanol production: Sono-assisted acid hydrolysis approach. Bioresour. Technol. 2011, 102, 7119–7123. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, M.N.; Ansari, S. Thermally reduced graphene oxide/thermoplastic polyurethane nanocomposites as photomechanical actuators. Adv. Mater. Lett. 2013, 4, 927–932. [Google Scholar]
- Kellici, S.; Acord, J.; Ball, J.; Reehal, H.S.; Morgan, D.; Saha, B. A single rapid route for the synthesis of reduced graphene oxide with antibacterial activities. RSC Adv. 2014, 4, 14858–14861. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Mungse, H.P.; Kumar, N.; Choudhary, S.; Jain, S.L.; Sain, B.; Khatri, O.P. Graphene oxide: An efficient and reusable carbocatalyst for aza-Michael addition of amines to activated alkenes. Chem. Commun. 2011, 47, 12673–12675. [Google Scholar] [CrossRef]
- Satish, B.; Venkata Rao, K.; Shilpa Chakra, C.H.; Tejaswi, T. Synthesis and characterization of graphene oxide and its antimicrobial activity against klebseilla and staphylococus. Int. J. Adv. Biotechnol. Res. 2013, 4, 142–146. [Google Scholar]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Kusurkar, T.S.; Maurya, S.K.; Meena, S.K.; Singh, S.K.; Sethy, N.; Bhargava, K.; Sharma, R.K.; Goswami, D.; Sarkar, S.; et al. Graphene oxide from silk cocoon: A novel magnetic fluorophore for multi-photon imaging. 3 Biotech 2014, 4, 67–75. [Google Scholar]
- Bo, Z.; Shuai, X.; Mao, S.; Yang, H.; Qian, J.; Chen, J.; Yan, J.; Cen, K. Green preparation of reduced graphene oxide for sensing and energy storage applications. Sci. Rep. 2014, 4, 1–5. [Google Scholar]
- Mao, S.; Pu, H.; Chen, J. Graphene oxide and its reduction: Modeling and experimental progress. RSC Adv. 2012, 2, 2643–2662. [Google Scholar] [CrossRef]
- Mao, S.; Yu, K.; Cui, S.; Bo, Z.; Lu, G.; Chen, J. A new reducing agent to prepare single-layer, high-quality reduced graphene oxide for device applications. Nanoscale 2011, 3, 2849–2853. [Google Scholar] [CrossRef] [PubMed]
- Vidano, R.P.; Fischbach, D.B.; Willis, L.J.; Loehr, T.M. Observation of Raman band shifting with excitation wavelength for carbons and graphites. Solid State Commun. 1981, 39, 341–344. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Chun, O.W.; Chen, M.L.; Zhang, K.; Zhang, F.J. The effect of thermal and ultrasonic treatment on the formation of graphene-oxide nanosheets. J. Korean Phys. Soc. 2010, 56, 1097–1102. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A. Visualization of defect densities in reduced graphene oxide. Carbon 2012, 50, 3666–3673. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somanathan, T.; Prasad, K.; Ostrikov, K.; Saravanan, A.; Krishna, V.M. Graphene Oxide Synthesis from Agro Waste. Nanomaterials 2015, 5, 826-834. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020826
Somanathan T, Prasad K, Ostrikov K, Saravanan A, Krishna VM. Graphene Oxide Synthesis from Agro Waste. Nanomaterials. 2015; 5(2):826-834. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020826
Chicago/Turabian StyleSomanathan, Thirunavukkarasu, Karthika Prasad, Kostya (Ken) Ostrikov, Arumugam Saravanan, and Vemula Mohana Krishna. 2015. "Graphene Oxide Synthesis from Agro Waste" Nanomaterials 5, no. 2: 826-834. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020826