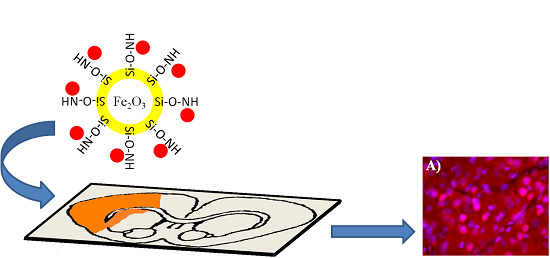
Targeting of Apoptotic Cells Using Functionalized Fe2O3 Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Morphology

2.2. Conjugation to SR-FLIVO


2.3. TUNEL Staining

2.4. Intracellular Tracking



3. Experimental Section
3.1. Synthesis and Surface Modification of NPs
3.2. Conjugation of FNPs with SR-FLIVO

3.3. In Vivo Study
3.4. In Vitro Tracking
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- David, L.V.; Stanley, J.K. Cell death in development. Cell 1999, 96, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, M.; Park, H.; Kim, J.H.; Kim, S.; Chung, H.; Choi, K.; Kim, I.S.; Seong, B.L.; Kwon, I.C. Cell-permeable and biocompatible polymeric nanoparticles for apoptosis imaging. J. Am. Chem. Soc. 2006, 128, 3490–3491. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.J.; Williams, B.W.; John, B.A.; Bischof, C.; Olin, M.; Gary, L.; Johnson, B.S.; Lee, B.W. Use of a fluorescently labeled poly-caspase inhibitor for in vivo detection of apoptosis related to vascular-targeting agent arsenic trioxide for cancer therapy. Technol. Cancer Res. Treat. 2007, 6, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Dicker, D.T.; Kim, S.H.; Jin, Z.; El-Deiry, W.S. Heterogeneity in non-invasive detection of apoptosis among human tumor cell lines using annexin-V tagged with EGFP or Qdot-705. Cancer Biol. Ther. 2005, 4, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.R.; Constantinescu, A.; Schlissel, M.S. Annexin V binds to positively selected B cells. J. Immunol. 2001, 166, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Tait, J.F.; Smith, C.; Wood, B.L. Measurement of phosphatidylserine exposure in leukocytes and platelets by whole-blood flow cytometry with annexin V. Blood Cells Mol. Dis. 1999, 25, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.G. Apoptosis: From morphological types of cell death to interacting pathways. Trends Pharmacol. Sci. 2002, 23, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.B.; Sexton, K.B.; Bogyo, M. Commonly used caspase inhibitors designed based on substrate specificity profiles lack selectivity. Cell Res. 2006, 16, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Lavaee, P.; Ramezani, M.; Abnous, K. Reversible targeting and controlled release delivery of daunorubicin to cancer cells by aptamer-wrapped carbon nanotubes. Eur. J. Pharm. Biopharm. 2011, 77, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Suriamoorthy, P.; Zhang, X.; Hao, G.; Joly, A.G.; Singh, S.; Hossu, M.; Sun, X.; Chen, W. Folic acid-CdTe quantum dot conjugates and their applications for cancer cell targeting. Cancer Nanotechnol. 2010, 1, 19–28. [Google Scholar] [CrossRef]
- Huang, X.; Jain, P.K.; El-Sayed, I.H.; El-Sayed, M.A. Gold nanoparticles: Interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2007, 2, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Styliano, P.T.; Cui, J.; Martin, J.; Chauhan, V.P. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Schleich, N.; Po, C.; Jacobs, D.; Ucakar, B.; Gallez, B. Comparison of active, passive and magnetic targeting to tumors of multifunctional paclitaxel/SPIO-loaded nanoparticles for tumor imaging and therapy. J. Control. Release 2014, 194, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Iv, M.; Telischak, N.; Feng, D.; Holdsworth, S.J.; Yeom, K.W.; Daldrup-Link, H.E. Clinical applications of iron oxide nanoparticles for magnetic resonance imaging of brain tumors. Nanomedicine 2015, 10, 993–1018. [Google Scholar] [CrossRef] [PubMed]
- Hanini, A.; Schmitt, A.; Kacem, K.; Chau, F.; Ammar, S.; Gavard, J. Evaluation of iron oxide nanoparticle compatibility. Int. J. Nanomed. 2011, 6, 787. [Google Scholar]
- Peng, X.H.; Qian, X.; Wang, A.Y.; Chen, Z.; Nie, S.; Shin, D.M. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. Int. J. Nanomed. 2008, 3, 311–321. [Google Scholar]
- Schleich, N.; Danhier, F.; Préat, V. Iron oxide-loaded nanotheranostics: Major obstacles to in vivo studies and clinical translation. J. Control. Release 2015, 198, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Khandhar, A.P.; Ferguson, R.M.; Krishnan, K.M. Monodispersed magnetite nanoparticles optimized for magnetic fluid hyperthermia: Implications in biological systems. J. App. Phys. 2011, 109, 07B310. [Google Scholar] [CrossRef]
- Tartaj, P. Encyclopedia of Nanoscience and Nanotechnology; American Scientific Publishers: Stevenson Ranch, CA, USA, 2003; Volume 6, p. 823. [Google Scholar]
- Atta, M.A.; Al-Lohedan, H.A.; Al-Hussain, S.A. Synthesis of stabilized myrrh-capped hydrocolloidal magnetite nanoparticles. Molecules 2014, 19, 11263–11278. [Google Scholar] [CrossRef] [PubMed]
- Cornel, R.M.; Schwertmann, U. The Iron Oxides, Structure, Properties, Reactions and Uses; VCH: Weinheim, Germany, 1996. [Google Scholar]
- Lockman, P.R.; Koziara, J.M.; Mumper, R.J.; Allen, D.D. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J. Drug Target. 2004, 12, 635–641. [Google Scholar] [CrossRef] [PubMed]
- ImmunoChemistry Technologies LLC. Available online: http://www.immunochemistry.com/FLIVO.htm (accessed on 2 April 2014).
- Smith, S.N.; Steer, R.P. The photophysics of Lissamine rhodamine-B sulfonyl chloride in aqueous solution: Implications for fluorescent protein—Dye conjugates. J. Photochem. Photobiol. A 2001, 139, 151–156. [Google Scholar] [CrossRef]
- Neves, C.; Byk, G.; Escriou, V.; Bussone, F.; Scherman, D.; Wils, P. Novel method for covalent fluorescent labeling of plasmid DNA that maintains structural integrity of the plasmid. Bioconjug. Chem. 2000, 11, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Watanabe, J.; Mekawy, M.M.; Fujiwara, R.; Morita, K.; Yamashita, T.; Amino, Y.; Chen, Y.; Logudurai, R.; Teramae, N. Extraction mechanisms of charged organic dye molecules into silica-surfactant nanochannels in a porous alumina membrane. Anal. Chim. Acta 2006, 556, 157–163. [Google Scholar] [CrossRef] [PubMed]
- El-Gamel, N.E.; Wortmann, L.; Arroub, K.; Mathur, S. Surface immobilization and release of sparfloxacin drug from SiO2@Fe2O3 core-shell nanoparticles. Chem. Commun. 2011, 47, 10076. [Google Scholar] [CrossRef]
- Shimizu, I.; Okabayashi, H.; Taga, K.; Nishio, E.; O’Connor, C.J. Diffuse reflectance infrared Fourier transform spectral study of the thermal and adsorbed-water effects of a 3-aminopropyltriethoxysilane layer modified onto the surface of silica gel. Vib. Spectosc. 1997, 14, 113–123. [Google Scholar] [CrossRef]
- Zhoua, J.; Leuschnerb, C.; Kumarc, C.; Hormesc, J.F.; Soboyejo, W.O. Sub-cellular accumulation of magnetic nanoparticles in breast tumors and metastases. Biomaterials 2006, 27, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Jendelová, P.; Herynek, V.; Urdzíková, L.; Glogarová, K.; Kroupová, J.; Andersson, B.; Bryja, V.; Burian, M.; Hájek, M.; Syková, E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J. Neurosci. Res. 2004, 76, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.B.; Yaacob, I.I. Synthesis and characterization of magnetic iron oxide nanoparticles via w/o microemulsion and Massart’s procedure. J. Mater. Process. Technol. 2007, 191, 235–237. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Ota, T.; Matsuoka, Y.; Tooyama, I.; Kimura, H.; Shimohama, S.; Nomura, Y.; Gebicke-Haerter, P.J.; Taniguchi, T. Hydrogen peroxide-induced apoptosis mediated by p53 protein in glial cells. GLIA 1999, 25, 154–164. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekawy, M.; Saito, A.; Shimizu, H.; Tominaga, T. Targeting of Apoptotic Cells Using Functionalized Fe2O3 Nanoparticles. Nanomaterials 2015, 5, 874-884. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020874
Mekawy M, Saito A, Shimizu H, Tominaga T. Targeting of Apoptotic Cells Using Functionalized Fe2O3 Nanoparticles. Nanomaterials. 2015; 5(2):874-884. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020874
Chicago/Turabian StyleMekawy, Moataz, Atsushi Saito, Hiroaki Shimizu, and Teiji Tominaga. 2015. "Targeting of Apoptotic Cells Using Functionalized Fe2O3 Nanoparticles" Nanomaterials 5, no. 2: 874-884. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020874