Kinetic Uptake Studies of Powdered Materials in Solution
Abstract
:1. Introduction
2. Results and Discussion

2.1. Dye Uptake Kinetics Equations and Models
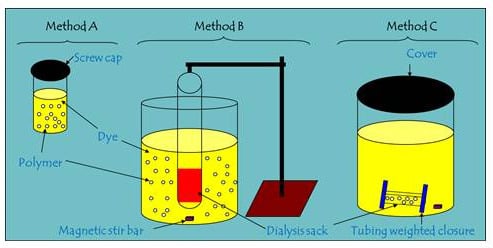
2.2. Comparison of Batch and One-pot Kinetic Methods

2.3. Comparison of Sorbent Materials with Phenolic Dyes
| Copolymer | Method A | Method C | ||
|---|---|---|---|---|
| Qe (μmol/g) | k1 (s−1) | Qe (μmol/g) | k1 (s−1) | |
| HDI-2 | 12.8 | 237 | 19.2 | 0.875 |
| CDI-1 | 11.6 | 100 | 3.13 | 0.978 |
| MDI-1 | 11.4 | 103 | 14.7 | 1.02 |
| PDI-1 | 3.21 | 156 | 2.17 | 0.54 |
| NDI-1 | 13.4 | 330 | 36.9 | 1.23 |

2.4. Comparison of Sorbent Materials with Phenolic Dyes at Variable Temperature
| Temperature | HDI-2 | MDI-1 | ||
|---|---|---|---|---|
| Qe (μmol/g) | k1 (s−1) | Qe (μmol/g) | k1 (s−1) | |
| 25 °C | 19.2 | 0.875 | 14.7 | 1.02 |
| 30 °C | 14.8 | 0.886 | 17.2 | 1.17 |
| 40 °C | 14.9 | 0.581 | 18.1 | 1.18 |

3. Experimental Section
3.1. Materials
3.2. Kinetic Uptake Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sparks, D.L.; Rechcigl, J.E. Comparison of batch and miscible displacement techniques to describe potassium adsorption kinetics in delaware soils. Soil Sci. Soc. Am. J. 1982, 46, 875–877. [Google Scholar] [CrossRef]
- Bermúdez-Couso, A.; Fernández-Calviño, D.; Rodríguez-Salgado, I.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Comparison of batch, stirred flow chamber, and column experiments to study adsorption, desorption and transport of carbofuran within two acidic soils. Chemosphere 2012, 88, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Scientific Committee on Plants-Plant Protection Products. Opinion on Methods for the Determination of the Organic Carbon Adsorption Coefficient (Koc) for a Plant Protection Product Active Substance in the Context of Council Directive 91/414/eec; European Commission: Brussel, Belgium, 2002; p. 12. [Google Scholar]
- Huang, H.; Fan, Y.; Wang, J.; Gao, H.; Tao, S. Adsorption kinetics and thermodynamics of water-insoluble crosslinked β-cyclodextrin polymer for phenol in aqueous solution. Macromol. Res. 2013, 21, 726–731. [Google Scholar] [CrossRef]
- Liu, H.; Cai, X.; Wang, Y.; Chen, J. Adsorption mechanism-based screening of cyclodextrin polymers for adsorption and separation of pesticides from water. Water Res. 2011, 45, 3499–3511. [Google Scholar] [CrossRef] [PubMed]
- Piccin, J.S.; Dotto, G.L.; Vieira, M.L.G.; Pinto, L.A.A. Kinetics and mechanism of the food dye FD&C red 40 adsorption onto chitosan. J. Chem. Eng. Data 2011, 56, 3759–3765. [Google Scholar]
- Azizian, S. Kinetic models of sorption: A theoretical analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Wilson, L.; Sammynaiken, R. Sorptive uptake studies of an aryl-arsenical with iron oxide composites on an activated carbon support. Materials 2014, 7, 1880–1898. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V.; Peru, K.M. Investigation of the sorption properties of β-cyclodextrin-based polyurethanes with phenolic dyes and naphthenates. J. Colloid Interface Sci. 2011, 356, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapsakad. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Wilson, L.D.; Mohamed, M.H.; Headley, J.V. Novel materials for environmental remediation of oil sands contaminants. Rev. Environ. Health 2014, 29, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Adsorption of Lead on Single and Mixed Solid Systems. Ph.D. Thesis, Western Michigan University, Ann Arbor, MI, USA, December 2007. [Google Scholar]
- Skoog, D.A.; West, D.M.; Holler, F.J.; Crouch, S.R. Fundamentals of Analytical Chemistry; Brooks/Cole: Belmont, CA, USA, 2014. [Google Scholar]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (sbse), a novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Ito, R.; Saito, K.; Nakazawa, H. Novel stir bar sorptive extraction methods for environmental and biomedical analysis. J. Pharm. Biomed. Anal. 2006, 40, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Design and characterization of novel β-cyclodextrin based copolymer materials. Carbohydr. Res. 2011, 346, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Atkins, P.; de Paula, J. Physical Chemistry; OUP Oxford: New York, NY, USA, 2014. [Google Scholar]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Estimation of the surface accessible inclusion sites of β-cyclodextrin based copolymer materials. Carbohydr. Polym. 2010, 80, 186–196. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Pratt, D.Y.; Guo, R.; Wu, C.; Headley, J.V. Evaluation of the accessible inclusion sites in copolymer materials containing β-cyclodextrin. Carbohydr. Polym. 2012, 87, 1241–1248. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Tuning the physicochemical properties of β-cyclodextrin based polyurethanes via cross-linking conditions. Microporous Mesoporous Mater. 2015, 214, 23–31. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Dolatkhah, A.; Aboumourad, T.; Dehabadi, L.; Wilson, L.D. Investigation of templated and supported polyaniline adsorbent materials. RSC Adv. 2015, 5, 6976–6984. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.H.; Wilson, L.D. Kinetic Uptake Studies of Powdered Materials in Solution. Nanomaterials 2015, 5, 969-980. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020969
Mohamed MH, Wilson LD. Kinetic Uptake Studies of Powdered Materials in Solution. Nanomaterials. 2015; 5(2):969-980. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020969
Chicago/Turabian StyleMohamed, Mohamed H., and Lee D. Wilson. 2015. "Kinetic Uptake Studies of Powdered Materials in Solution" Nanomaterials 5, no. 2: 969-980. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020969