Nano-Sized Cyclodextrin-Based Molecularly Imprinted Polymer Adsorbents for Perfluorinated Compounds—A Mini-Review
Abstract
:1. Introduction

2. CD/PFC Host-Guest Chemistry
3. Adsorbents for PFCs
3.1. NIP Adsorbents
| Molecular Formula and Structure * | PFOA | PFOS | PFBA |
| C8HF15O2 | C8HF17O3S | C4HF7O2 | |
 | |||
| Molecular weight (g/moL) | 414 | 500 | 214 |
| Solubility (g/L, 25 °C) | 3.4 a | 0.57 | High |
| Melting Point (°C) | 45–55 | 45–54 | −17.5 |
| Boiling Point (°C) | 188 | 188–192 | 120 |
| pKa | 2.5 | 0.14 | 0.08–0.4 |
| cmc (mmol/L) | 8.5–10 | 2.0 | No data |
| Vapor pressure (mmHg, 25 °C) | 0.017 | 2.48 × 10−6 | 10 |
3.2. Conventional MIP Adsorbents




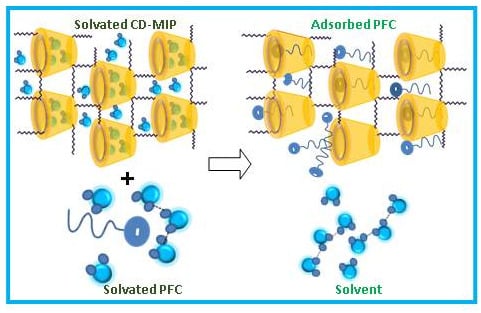
3.3. CD-MIP Adsorbents




4. Conclusions and Future Perspectives
- Size-fit complementarity between the host binding sites and the guest at the nanometer scale and beyond;
- Introduction of high affinity functional groups such as hydroxyl and ethers that possess high polarizability and greater affinity than graphene or carbonaceous materials that possess reduced levels of surface functional heteroatom groups; and
- Greater ease of sorbent regeneration because relatively weak interactions are involved.
Acknowledgements
Author Contributions
Conflicts of Interest
References
- The Basel, Rotterdam and Stockholm Conventions on Chemicals and Wastes—Regulation, Sound Management and Governance. In Trade, the Environment and Technology Transfer; Petitpierre-Sauvain, A. (Ed.) EcoLomics International: Geneva, Switzerland, 2008.
- Fujii, S.; Polprasert, C.; Tanaka, S.; Lien, N.P.H.; Qiu, Y. New POPs in the water environment: Distribution, bioaccumulation and treatment of perfluorinated compounds—A review paper. J. Water Supply Res. Technol.-AQUA 2007, 56, 313–326. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Perfluorochemical surfactants in the environment. Environ. Sci. Technol. 2002, 36, 146A–152A. [Google Scholar] [CrossRef] [PubMed]
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2012, 36, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.S.C.; Szostek, B.; DeRito, C.M.; Madsen, E.L. Investigating the biodegradability of perfluorooctanoic acid. Chemosphere 2010, 80, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.M.; Suchan, M.; Gerstmann, S.; Frank, H. Perfluorooctanoic acid and perfluorooctane sulfonate released from a waste water treatment plant in Bavaria, Germany. Environ. Sci. Pollut. Res. 2010, 17, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater—A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanjuan, M.; Meyer, J.; Damásio, J.; Faria, M.; Barata, C.; Lacorte, S. Screening of Perfluorinated Chemicals (PFCs) in Various Aquatic Organisms. Anal. Bioanal. Chem. 2010, 398, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, K.; Zakaria, M.P.; Al-Odaini, N.A.; Bakhtiari, A.R.; Latif, P.A. Perfluorooctanoic Acid (PFOA) and Perfluorooctane Sulfonate (PFOS) in Surface Water From the Langat River, Peninsular Malaysia. Environ. Forensics 2012, 13, 82–92. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Maternal levels of perfluorinated chemicals and subfecundity. Hum. Reprod. 2009, 24, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Butenhoff, J.L.; Rogers, J.M. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmacol. 2004, 198, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.; Thibodeaux, J.R.; Hanson, R.G.; Narotsky, M.G.; Rogers, J.M.; Lindstrom, A.B.; Strynar, M.J. Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci. 2006, 90, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Butenhoff, J.L.; Kennedy, G.L.; Frame, S.R.; O’Connor, J.C.; York, R.G. The reproductive toxicology of ammonium perfluorooctanoate (APFO) in the rat. Toxicology 2004, 196, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Case, M.T.; York, R.G.; Christian, M.S. Rats and rabit oral developmental taxicological studies with two perfluorinated compounds. Int. J. Toxicol. 2001, 20, 101–109. [Google Scholar] [PubMed]
- Kawashima, Y.; Kobayashi, H.; Miura, H.; Kozuka, H. Characterization of hepatic responses of rat to administration of perfluorooctanoic and perfluorodecanoic acids at low levels. Toxicology 1995, 99, 169–178. [Google Scholar] [CrossRef]
- Kudo, N.; Iwase, Y.; Okayachi, H.; Yamakawa, Y.; Kawashima, Y. Induction of hepatic peroxisome proliferation by 8-2 telomer alcohol feeding in mice: Formation of perfluorooctanoic acid in the liver. Toxicol. Sci. 2005, 86, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Abedi-Valugerdi, M.; Xie, Y.; Zhao, X.Y.; Möller, G.; Dean Nelson, B.; DePierre, J.W. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int. Immunopharmacol. 2002, 2, 389–397. [Google Scholar] [CrossRef]
- Peden-Adams, M.M.; Keller, J.M.; Eudaly, J.G.; Berger, J.; Gilkeson, G.S.; Keil, D.E. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci. 2008, 104, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Dewitt, J.C.; Copeland, C.B.; Luebke, R.W. Suppression of humoral immunity by perfluorooctanoic acid is independent of elevated serum corticosterone concentration in mice. Toxicol. Sci. 2009, 109, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Guruge, K.S.; Yeung, L.W.Y.; Yamanaka, N.; Miyazaki, S.; Lam, P.K.S.; Giesy, J.P.; Jones, P.D.; Yamashita, N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol. Sci. 2006, 89, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, G.L.; Butenhoff, J.L.; Olsen, G.W.; O’Connor, J.C.; Seacat, A.M.; Perkins, R.G.; Biegel, L.B.; Murphy, S.R.; Farrar, D.G. The toxicology of perfluorooctanoate. Crit. Rev. Toxicol. 2004, 34, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Gilliland, F.D.; Mandel, J.S. Mortality among employees of a perfluorooctanoic acid production plant. J. Occup. Environ. Med. 1993, 35, 950–954. [Google Scholar] [CrossRef]
- Vierke, L.; Staude, C.; Biegel-Engler, A.; Drost, W.; Schulte, C. Perfluorooctanoic acid (PFOA)-main concerns and regulatory developments in Europe from an environmental point of view. Environ. Sci. Eur. 2012, 24, 1–11. [Google Scholar] [CrossRef]
- Yang, K.-H.; Lin, Y.-C.; Fang, M.-D.; Wu, C.-H.; Panchangam, S.C.; Hong, P.-K.A.; Lin, C.-F. Sorption of perfluorooctanoic acid (PFOA) onto sediment in the presence of dissolved natural organics. Sep. Sci. Technol. 2013, 48, 1473–1478. [Google Scholar] [CrossRef]
- Giesy, J.P.; Naile, J.E.; Khim, J.S.; Jones, P.D.; Newsted, J.L. Aquatic toxicology of perfluorinated chemicals. Rev. Environ. Contam. Toxicol. 2010, 202, 1–52. [Google Scholar] [PubMed]
- Kissa, E. Fluorinated Surfacts and Repellents, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Ding, G.; Peijnenburg, W.J.G.M. Physicochemical properties and aquatic toxicity of poly-and perfluorinated compounds. Crit. Rev. Environ. Sci. Technol. 2013, 43, 598–678. [Google Scholar] [CrossRef]
- Stahl, T.; Mattern, D.; Brunn, H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011, 23, 1–52. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, Y.; Wang, J.; Cai, Y. Distribution of perfluorinated compounds in water, sediment, biota and floating plants in Baiyangdian Lake, China. J. Environ. Monit. 2012, 14, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Kwadijk, C.J.A.F.; Korytár, P.; Koelmans, A.A. Distribution of perfluorinated compounds in aquatic systems in the netherlands. Environ. Sci. Technol. 2010, 44, 3746–3751. [Google Scholar] [CrossRef] [PubMed]
- Naile, J.E.; Khim, J.S.; Hong, S.; Park, J.; Kwon, B.O.; Ryu, J.S.; Hwang, J.H.; Jones, P.D.; Giesy, J.P. Distributions and bioconcentration characteristics of perfluorinated compounds in environmental samples collected from the west coast of Korea. Chemosphere 2013, 90, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Fung, B.M.; Christian, S.D. NMR study of cyclodextrin inclusion of fluorocarbon surfactants in solution. Langmuir 1992, 8, 446–451. [Google Scholar] [CrossRef]
- Xing, H.; Lin, S.-S.; Yan, P.; Xiao, J.-X.; Chen, Y.-M. NMR studies on selectivity of beta-cyclodextrin to fluorinated/hydrogenated surfactant mixtures. J. Phys. Chem. B 2007, 111, 8089–8095. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.D.; Verrall, R.E. A 1H NMR study of cyclodextrin-hydrocarbon surfactant inclusion complexes in aqueous solutions. Can. J. Chem. 1998, 76, 25–34. [Google Scholar]
- Karoyo, A.H.; Borisov, A.S.; Wilson, L.D.; Hazendonk, P. Formation of host-guest complexes of β-cyclodextrin and perfluorooctanoic acid. J. Phys. Chem. B 2011, 115, 9511–9527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hogen-Esch, T.E.; Boschet, F.; Margaillan, A.; Boschet, F.; Margaillan, A. Complex formation of cyclodextrin- and perfluorocarbon- modified water-soluble polymers. Langmuir 1998, 14, 4972–4977. [Google Scholar] [CrossRef]
- Palepu, R.; Richardson, J.E.; Reinsborough, V.C. Binding constants of .beta.-cyclodextrin/surfactant Inclusion by conductivity measurements. Langmuir 1989, 5, 218–221. [Google Scholar] [CrossRef]
- Palepu, R.; Reinsborough, V.C. Solution inclusion complexes of cyclodextrins with sodium perfluorooctanoate. Can. J. Chem. 1989, 67, 1550–1553. [Google Scholar] [CrossRef]
- Saint Aman Eric, S.D. A conductimetric study of the association between cyclodextrins and surfactants-application to the electrochemical study of a mixed aqueous system: Substrate, cyclodextrin, surfactant. J. Colloid Interface Sci. 1990, 138, 365–375. [Google Scholar] [CrossRef]
- Junquera, E.; Tardajos, G.; Aicart, E. Effect of the presence of β-cyclodextrin on the micellization process of sodium dodecyl or sodium perfluoroctanoate in water. Langmuir 1993, 9, 1213–1219. [Google Scholar] [CrossRef]
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents—A review. J. Hazard. Mater. 2014, 274, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Ternes, T. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2014, 81, 4645–4677. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Takagi, Y.; Tanaka, M.; Tsuruho, K.; Okitsu, K.; Maeda, Y. Sonochemical decomposition of perfluorooctane sulfonate and perfluorooctanoic acid. Environ. Sci. Technol. 2005, 39, 3388–3392. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Noma, Y.; Sakai, S.I.; Shibata, Y. Photodegradation of perfluorooctane sulfonate by UV irradiation in water and alkaline 2-propanol. Environ. Sci. Technol. 2007, 41, 5660–5665. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, J.; Zhao, G.; Fan, J.; Wang, Y.; Wang, Y. Fabrication of novel SnO2-Sb/carbon aerogel electrode for ultrasonic electrochemical oxidation of perfluorooctanoate with high catalytic efficiency. Appl. Catal. B Environ. 2013, 136–137, 278–286. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; Sierra-Alvarez, R. Removal of perfluorinated surfactants by sorption onto granular activated carbon, zeolite and sludge. Chemosphere 2008, 72, 1588–1593. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, R.; Deng, S.; Huang, J.; Yu, G. Sorption of perfluorooctane sulfonate and perfluorooctanoate on activated carbons and resin: Kinetic and isotherm study. Water Res. 2009, 43, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, C.; Li, F.; Bo, X.; Liu, G.; Zhou, Q. Equilibrium and kinetics study on the adsorption of perfluorooctanoic acid from aqueous solution onto powdered activated carbon. J. Hazard. Mater. 2009, 169, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.C.; Børresen, M.H.; Schlabach, M.; Cornelissen, G. Sorption of perfluorinated compounds from contaminated water to activated carbon. J. Soils Sediments 2010, 10, 179–185. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Quan, X.; Zhang, Y. Enhanced adsorption of PFOA and PFOS on multiwalled carbon nanotubes under electrochemical assistance. Environ. Sci. Technol. 2011, 45, 8498–8505. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.E.; Farrell, J. Removal of perfluorooctane and perfluorobutane sulfonate from water via carbon adsorption and ion exchange. Sep. Sci. Technol. 2010, 45, 762–767. [Google Scholar] [CrossRef]
- Senevirathna, S.T.M.L.D.; Tanaka, S.; Fujii, S.; Kunacheva, C.; Harada, H.; Shivakoti, B.R.; Okamoto, R. A comparative study of adsorption of perfluorooctane sulfonate (PFOS) onto granular activated carbon, ion-exchange polymers and non-ion-exchange polymers. Chemosphere 2010, 80, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Cheng, J.; Vecitis, C.D.; Hoffmann, M.R. Sorption of perfluorochemicals to granular activated carbon in the presence of ultrasound. J. Phys. Chem. A 2011, 115, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, Q.; Nie, Y.; Wei, H.; Wang, B.; Huang, J.; Yu, G.; Xing, B. Sorption mechanisms of perfluorinated compounds on carbon nanotubes. Environ. Pollut. 2012, 168, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chularueangaksorn, P.; Tanaka, S.; Fujii, S.; Kunacheva, C. Batch and column adsorption of perfluorooctane sulfonate on anion exchange resins and granular activated carbon. J. Appl. Polym. Sci. 2014, 131, 39782–39788. [Google Scholar] [CrossRef]
- Yao, Y.; Volchek, K.; Brown, C.E.; Robinson, A.; Obal, T. Comparative study on adsorption of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) by different adsorbents in water. Water Sci. Technol. 2014, 70, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; Lelyveld, T.P.; Mavrofidis, S.D.; Kingman, S.W.; Miles, N.J. Microwave heating applications in environmental engineering—a review. Resour. Conserv. Recycl. 2002, 34, 75–90. [Google Scholar] [CrossRef]
- Salvador, F.; Jiménez, C.S. A new method for regenerating activated carbon by thermal desorption with liquid water under subcritical conditions. Carbon N. Y. 1996, 34, 511–516. [Google Scholar] [CrossRef]
- Chen, M.S.; Wang, J.H. Microwave regeneration of activated carbon containing toluene. Environ. Pollut. Control Technol. Equip. 2006, 7, 77–79. [Google Scholar]
- Zhang, H.; Ye, L.; Zhong, H. Regeneration of phenol-saturated activated carbon in an electrochemical reactor. J. Chem. Technol. Biotechnol. 2002, 77, 1246–1250. [Google Scholar] [CrossRef]
- Mundale, V.D.; Joglekar, H.S.; Kalam, A.; Joshi, J.B. Regeneration of spent activated carbon by wet air oxidation. Can. J. Chem. Eng. 1991, 69, 1149–1159. [Google Scholar] [CrossRef]
- Yu, J.G.; Zhao, X.H.; Yang, H.; Chen, X.H.; Yang, Q.; Yu, L.Y.; Jiang, J.H.; Chen, X.Q. Aqueous adsorption and removal of organic contaminants by carbon nanotubes. Sci. Total Environ. 2014, 482–483, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour. Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.N.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Crini, G.; Bertini, S.; Torri, G.; Naggi, A.; Sforzini, D.; Vecchi, C.; Janus, L.; Lekchiri, Y.; Morcellet, M. Sorption of aromatic compounds in water using insoluble cyclodextrin polymers. J. Appl. Polm. Sci. 1998, 68, 1973–1978. [Google Scholar] [CrossRef]
- Mamba, B.B.; Krause, R.W.; Malefetse, T.J.; Nxumalo, E.N. Monofunctionalized cyclodextrin polymers for the removal of organic pollutants from water. Environ. Chem. Lett. 2007, 5, 79–84. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin–epichlorohydrin polymers. Prog. Polym. Sci. 2012, 38, 344–368. [Google Scholar] [CrossRef]
- Murai, S.; Imajo, S.; Inumaru, H.; Takahashi, K.; Hattori, K. Adsorption and recovery of ionic surfactants by beta-cyclodextrin polymer. J. Colloid Interface Sci. 1997, 190, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Kakazu, M.; Shibata, M.; Hishiya, T.; Komiyama, M. Synthesis of molecularly imprinted polymers of beta-cyclodextrin for the efficient recognition of cholesterol. Supramol. Sci. 1998, 5, 417–421. [Google Scholar] [CrossRef]
- Van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-based polymeric materials: Synthesis, properties, and pharmaceutical/biomedical applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Design and characterization of novel β-cyclodextrin based copolymer materials. Carbohydr. Res. 2011, 346, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V.; Peru, K.M. Sequestration of naphthenic acids from aqueous solution using β-cyclodextrin-based polyurethanes. Phys. Chem. Chem. Phys. 2011, 13, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.Y.; Wilson, L.D.; Kozinski, J.A.; Mohart, A.M. Preparation and sorption studies of β-cyclodextrin/epichlorohydrin copolymers. J. Appl. Polym. Sci. 2010, 116, 2982–2989. [Google Scholar] [CrossRef]
- Wilson, L.D.; Guo, R. Preparation and sorption studies of polyester microsphere copolymers containing β-cyclodextrin. J. Colloid Interface Sci. 2012, 387, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.D.; Mohamed, M.H.; Headly, J.V. Novel materials fo environmental remediation of oil sands contaminants. Rev. Env. Heal. 2014, 29, 5–8. [Google Scholar]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J.; Osa, T. Comprehensive Supramolecular Chemistry; Pergamon: Oxford, UK, 1996; Volume 3. [Google Scholar]
- Karoyo, A.H.; Wilson, L.D. Tunable macromolecular-based materials for the adsorption of perfluorooctanoic and octanoic acid anions. J. Colloid Interface Sci. 2013, 402, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Palepu, R.; Reinsborough, V.C. Surfactant-yclodextrin interactions by conductance measurements. Can. J. Chem. 1988, 66, 325–328. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Szejtli, J. Utilization of cyclodextrins in industrial products and processes. J. Mater. Chem. 1997, 7, 575–587. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons, Ltd: West Sussex, UK, 2009. [Google Scholar]
- Parker, K.M.; Stalcup, A.M. Affinity capillary electrophoresis and isothermal titration calorimetry for the determination of fatty acid binding with beta-cyclodextrin. J. Chromatogr. A 2008, 1204, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Wenz, G. Cyclodextrins as building-blocks for supramolecular structures and functional units. Angew. Chem. Int. Ed. Engl. 1994, 33, 803–822. [Google Scholar] [CrossRef]
- Qaqish, S.E.; Urquhart, S.G.; Lanke, U.; Brunet, S.M.K.; Paige, M.F. Phase separation of palmitic acid and perfluorooctadecanoic acid in mixed langmuir-blodgett monolayer films. Langmuir 2009, 25, 7401–7409. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, T.; Amada, K.; Miyagishi, S. Micellar immiscibility of lithium lithium tetradecyl sulfate mixture. 1997, 7463, 4569–4573. [Google Scholar]
- Pesek, J.J.; Matyska, M.T. SPE Sorbents and Formats. In Solid-Phase Extraction: Principles, Techniques, and Applications; Taylor & Francis Group LLC.: Florence, SC, USA, 2000; pp. 1–20. [Google Scholar]
- Zhang, Q.; Deng, S.; Yu, G.; Huang, J. Removal of perfluorooctane sulfonate from aqueous solution by crosslinked chitosan beads: Sorption kinetics and uptake mechanism. Bioresour. Technol. 2011, 102, 2265–2271. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zheng, Y.Q.; Xu, F.J.; Wang, B.; Huang, J.; Yu, G. Highly efficient sorption of perfluorooctane sulfonate and perfluorooctanoate on a quaternized cotton prepared by atom transfer radical polymerization. Chem. Eng. J. 2012, 193–194, 154–160. [Google Scholar] [CrossRef]
- Deng, S.; Niu, L.; Bei, Y.; Wang, B.; Huang, J.; Yu, G. Adsorption of perfluorinated compounds on aminated rice husk prepared by atom transfer radical polymerization. Chemosphere 2013, 91, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Burns, D.C.; Ellis, D.A.; Li, H.; Mcmurdo, C.J.; Webster, E. Experimental pKa determination for perfluorooctanoic acid (PFOA) and the potential impact of pKa concentration dependence on laboratory-measured partitioning phenomena and environmental modeling. Environ. Sci. Technol. 2008, 42, 9283–9288. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.; Würtz, J. Unusual phenomena in perfluorosurfactants. J. Mol. Liq. 1997, 72, 191–230. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Sidhu, P.; Wilson, L.D.; Hazendonk, P. Characterization and dynamic properties for the solid inclusion complexes of β-cyclodextrin and perfluorobutyric acid. J. Phys. Chem. C 2014, 118, 15460–15473. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Sun, H.; Yan, H.; Row, K.H. Molecularly imprinted polymers for solid phase extraction. Chromatographia 2006, 64, 625–634. [Google Scholar] [CrossRef]
- Lasáková, M.; Jandera, P. Molecularly imprinted polymers and their application in solid phase extraction. J. Sep. Sci. 2009, 32, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.A.; Andersson, H.S.; Nicholls, I.A. The rational use of hydrophobic effect-based recognition in molecularly imprinted polymers. J. Mol. Recognit. 1998, 11, 94–97. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Andersson, H.S.; Nicholls, I.A. Combined hydrophobic and electrostatic interaction-based recognition in molecularly imprinted polymers. Macromolecules 1999, 32, 633–636. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Andersson, H.S.; Nicholls, I.A. On the role of electrostatic interactions in the enantioselective recognition of phenylalanine in molecularly imprinted polymers incorporating β-cyclodextrin. Polym. J. 2005, 37, 793–796. [Google Scholar] [CrossRef]
- Song, S.H.; Shirasaka, K.; Katayama, M.; Nagaoka, S.; Yoshihara, S.; Osawa, T.; Sumaoka, J.; Asanuma, H.; Komiyama, M. Recognition of solution structures of peptides by molecularly imprinted cyclodextrin polymers. Macromolecules 2007, 40, 3530–3532. [Google Scholar] [CrossRef]
- Asanuma, H.; Hishiya, T.; Komiyama, M. Efficient separation of hydrophobic molecules by molecularly imprinted cyclodextrin polymers. J. Incl. Phenom. 2004, 50, 51–55. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, X.; Chen, Q. Separation processes in the presence of cyclodextrins using molecular imprinting technology and ionic liquid cooperating approach. Curr. Org. Chem. 2011, 15, 74–85. [Google Scholar] [CrossRef]
- Folch-Cano, C.; Yazdani-Pedram, M.; Olea-Azar, C. Inclusion and functionalization of polymers with cyclodextrins: Current applications and future prospects. Molecules 2014, 19, 14066–14079. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Lazaridis, N.K.; Bikiaris, D.N. Optimization of chitosan and β-cyclodextrin molecularly imprinted polymer synthesis for dye adsorption. Carbohydr. Polym. 2013, 91, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Deng, S.; Yu, G. Selective removal of perfluorooctane sulfonate from aqueous solution using chitosan-based molecularly imprinted polymer adsorbents. Water Res. 2008, 42, 3089–3097. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Shuai, D.; Yu, Q.; Huang, J.; Yu, G. Selective sorption of perfluorooctane sulfonate on molecularly imprinted polymer adsorbents. Front. Environ. Sci. Eng. China 2009, 3, 171–177. [Google Scholar] [CrossRef]
- Surikumaran, H.; Mohamad, S.; Sarih, N.M. Molecular imprinted polymer of methacrylic acid functionalised β-cyclodextrin for selective removal of 2,4-dichlorophenol. Int. J. Mol. Sci. 2014, 15, 6111–6136. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Kakazu, M.; Shibata, M.; Hishiya, T. Molecularly imprinted polymer of β-cyclodextrin for the efficient recognition of cholesterol. Chem. Commun. 1997, 5, 1971–1972. [Google Scholar] [CrossRef]
- Xu, Z.F.; Wen, G.; Kuang, D.Z.; Zhang, F.X.; Tang, S.P. Selective separation of deltamethrin by molecularly imprinted polymers using a β-cyclodextrin derivative as the functional monomer. J. Environ. Sci. Heal. B 2013, 48, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Akiyama, T.; Kajiya, K.; Hishiya, T.; Komiyama, M. Molecular imprinting of cyclodextrin in water for the recognition of nanometer-scaled guests. Anal. Chim. Acta 2001, 435, 25–33. [Google Scholar] [CrossRef]
- Qin, L.; He, X.W.; Li, W.Y.; Zhang, Y.K. Molecularly imprinted polymer prepared with bonded β-cyclodextrin and acrylamide on functionalized silica gel for selective recognition of tryptophan in aqueous media. J. Chromatogr. A 2008, 1187, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, B.; Muruganandham, M.; Suri, R.P.S. Development of high efficiency silica coated β-cyclodextrin polymeric adsorbent for the removal of emerging contaminants of concern from water. J. Hazard. Mater. 2014, 273, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Kida, T.; Takemine, S.; Matsumura, C.; Nakano, T.; Kuramitsu, M.; Adachi, K.; Akashi, M. Efficient removal and recovery of perfluorinated compounds from water by surface-tethered β-cyclodextrins on polystyrene particles. Chem. Lett. 2013, 42, 392–394. [Google Scholar] [CrossRef]
- Takayose, M.; Nishimoto, K.; Matsui, J. A fluorous synthetic receptor that recognizes perfluorooctanoic acid (PFOA) via fluorous interaction obtained by molecular imprinting. Analyst 2012, 137, 2762–2765. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Li, D. New organic nanoporous polymers and their inclusion complexes. Chem. Mater. 1999, 11, 872–874. [Google Scholar] [CrossRef]
- Karoyo, A.H. Structural Studies of Supramolecular Host-Guest Systems. Ph.D. Thesis, University of Saskatchewan, Saskatoon, Canada, 2014. [Google Scholar]
- Deng, W.; Yamaguchi, H.; Takashima, Y.; Harada, A. A chemical-responsive supramolecular hydrogel from modified cyclodextrins. Angew. Chem. Int. Ed. 2007, 46, 5144–5147. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Tunable polymeric sorbent materials for fractionation of model naphthenates. J. Phys. Chem. B 2013, 117, 3659–3666. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.H.; Wilson, L.D. Porous copolymer resins: Tuning pore structure and surface area with non reactive porogens. Nanomaterials 2012, 2, 163–186. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V. Tuning the physicochemical properties of β-cyclodextrin based polyurethanes via cross-linking conditions. Microporous Mesoporous Mater. 2015, 214, 23–31. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karoyo, A.H.; Wilson, L.D. Nano-Sized Cyclodextrin-Based Molecularly Imprinted Polymer Adsorbents for Perfluorinated Compounds—A Mini-Review. Nanomaterials 2015, 5, 981-1003. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020981
Karoyo AH, Wilson LD. Nano-Sized Cyclodextrin-Based Molecularly Imprinted Polymer Adsorbents for Perfluorinated Compounds—A Mini-Review. Nanomaterials. 2015; 5(2):981-1003. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020981
Chicago/Turabian StyleKaroyo, Abdalla H., and Lee D. Wilson. 2015. "Nano-Sized Cyclodextrin-Based Molecularly Imprinted Polymer Adsorbents for Perfluorinated Compounds—A Mini-Review" Nanomaterials 5, no. 2: 981-1003. https://0-doi-org.brum.beds.ac.uk/10.3390/nano5020981