Preparation and Characterization of Polyvinylpyrrolidone/Cellulose Nanocrystals Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNC
2.3. Preparation of PVP/CNC Composites
2.4. Characterization
2.4.1. Determination of Size and Surface Charge of CNC Particles
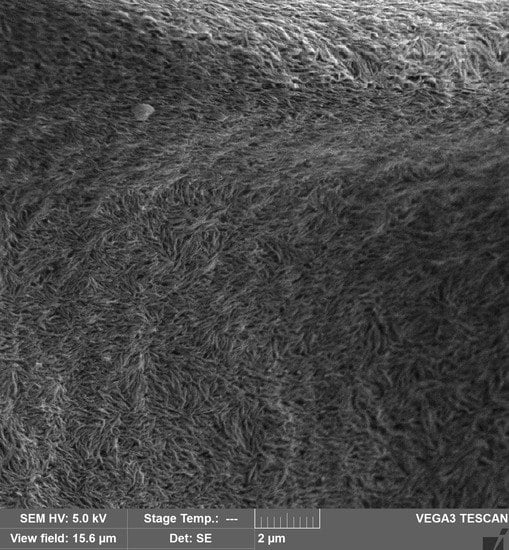
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.4. X-ray Diffraction Analysis
2.4.5. Thermogravimetric Analysis (TGA)
2.4.6. Differential Scanning Calorimetry (DSC)
2.4.7. Mechanical Properties
2.4.8. Water Uptake
3. Results and Discussion
3.1. FTIR Analysis
3.2. Water Adsorption upon the PVP/CNC Composites
3.3. Density of the PVP/CNC Composite Films
3.4. TG Analysis
3.5. DSC Analysis
3.6. Mechanical Properties
ηL = (Er − 1)/(Er + 2ρ),
ηT = (Er − 1)/(Er + 2),
3.7. X-ray Diffraction Analysis
3.8. Morphologies of the PVP/CNC Composites
3.9. PVP Adsorption onto CNC Particles
3.10. Dispersibility of Freeze-Dried PVP/CNC Composites in Water and Some Organic Solvents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Khattab, M.M.; Abdel-Hady, N.A.; Dahman, Y. Cellulose nanocomposites: Opportunities, challenges, and applications. In Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Abdul Khalil, H.P.S., Eds.; Woodhead Publishing: Toronto, ON, Canada, 2017; Chapter 21; pp. 483–516. ISBN 978-0-08-100957-4. [Google Scholar]
- Julkapli, N.M.; Bagheri, S. Progress on nanocrystalline cellulose biocomposites. React. Funct. Polym. 2017, 112, 9–21. [Google Scholar] [CrossRef]
- Tardy, B.L.; Yokota, S.; Ago, M.; Xiang, W.; Kondo, T.; Bordes, R.; Rojas, O.J. Nanocellulose–surfactant interactions. Curr. Opin. Colloid In. 2017, 29, 57–67. [Google Scholar] [CrossRef]
- Salas, C.; Nypelö, T.; Rodriguez-Abreu, C.; Carrillo, C.; Rojas, O.J. Nanocellulose properties and applications in colloids and interfaces. Curr. Opin. Colloid In. 2014, 19, 383–396. [Google Scholar] [CrossRef]
- Moreau, C.; Villares, A.; Capron, I.; Cathala, B. Tuning supramolecular interactions of cellulose nanocrystals to design innovative functional materials. Ind. Crop. Prod. 2016, 93, 96–107. [Google Scholar] [CrossRef]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid In. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Tang, J.; Sisler, J.; Grishkewich, N.; Tam, K.C. Functionalization of cellulose nanocrystals for advanced applications. J. Colloid Interf. Sci. 2017, 494, 397–409. [Google Scholar] [CrossRef]
- Niinivaara, E.; Faustini, M.; Tammelin, T.; Kontturi, E. Mimicking the Humidity Response of the Plant Cell Wall by Using Two-Dimensional Systems: The Critical Role of Amorphous and Crystalline Polysaccharides. Langmuir 2016, 32, 2032. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Blanco, A.; Negro, C. Study of The Reaction Mechanism to Produce Nanocellulose-Graft-Chitosan Polymer. Nanomaterials 2018, 8, 883. [Google Scholar] [CrossRef]
- Tenhunen, T.-M.; Pöhler, T.; Kokko, A.; Orelma, H.; Schenker, M.; Gane, P.; Tammelin, T. Enhancing the Stability of Aqueous Dispersions and Foams Comprising Cellulose Nanofibrils (CNF) with CaCO3 Particles. Nanomaterials 2018, 8, 651. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyrychenko, A.; Korsun, O.M.; Gubin, I.I.; Kovalenko, S.M.; Kalugin, O.N. Atomistic Simulations of Coating of Silver Nanoparticles with Poly(vinylpyrrolidone) Oligomers: Effect of Oligomer Chain Length. J. Phys. Chem. C 2015, 119, 7888–7899. [Google Scholar] [CrossRef]
- Al-Saidi, W.A.; Feng, H.; Fichthorn, K.A. Adsorption of Polyvinylpyrrolidone on Ag Surfaces: Insight into a Structure-Directing Agent. Nano Lett. 2012, 12, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Nathanael, A.J.; Seo, Y.H.; Oh, T.H. PVP Assisted Synthesis of Hydroxyapatite Nanorods with Tunable Aspect Ratio and Bioactivity. J. Nanomater. 2015, 621785. [Google Scholar] [CrossRef]
- Kadlubowski, S. Radiation-induced synthesis of nanogels based on poly(N-vinyl-2-pyrrolidone)—A review. Radiat. Phys. Chem. 2014, 102, 29–39. [Google Scholar] [CrossRef]
- An, J.-C.; Weaver, A.; Kim, B.; Barkatt, A.; Poster, D.; Vreeland, W.N.; Silverman, J.; Al-Sheikhly, M. Radiation-induced synthesis of poly(vinylpyrrolidone) nanogel. Polymer 2011, 52, 5746–5755. [Google Scholar] [CrossRef]
- Teodorescu, M.; Morariu, S.; Bercea, M.; Săcărescu, L. Viscoelastic and structural properties of poly(vinyl alcohol)/poly(vinylpyrrolidone) hydrogels. RSC Adv. 2016, 6, 39718–39727. [Google Scholar] [CrossRef]
- Nho, Y.C.; Park, K.R. Preparation and Properties of PVA/PVP Hydrogels Containing Chitosan by Radiation. J. Appl. Polym. Sci. 2002, 85, 1787–1794. [Google Scholar] [CrossRef]
- Yu, H.; Xu, X.; Chen, X.; Hao, J.; Jing, X. Medicated Wound Dressings Based on Poly(vinyl alcohol)/Poly(N-vinyl pyrrolidone)/Chitosan Hydrogels. J. Appl. Polym. Sci. 2006, 101, 2453–2463. [Google Scholar] [CrossRef]
- Halake, K.; Birajdar, M.; Kim, B.S.; Bae, H.; Lee, C.C.; Kim, Y.J.; Kim, S.; Kim, H.J.; Ahn, S.; An, S.Y.; Lee, J. Recent application developments of water-soluble synthetic polymers. J. Ind. Eng. Chem. 2014, 20, 3913–3918. [Google Scholar] [CrossRef]
- Nešić, A.; Ružić, J.; Gordić, M.; Ostojić, S.; Micić, D.; Onjia, A. Pectin-polyvinylpyrrolidone films: A sustainable approach to the development of biobased packaging materials. Compos. Part B-Eng. 2017, 110, 56–61. [Google Scholar] [CrossRef]
- Balgis, R.; Murata, H.; Goi, Y.; Ogi, T.; Okuyama, K.; Bao, L. Synthesis of Dual-Size Cellulose–Polyvinylpyrrolidone Nanofiber Composites via One-Step Electrospinning Method for High-Performance Air Filter. Langmuir 2017, 33, 6127–6134. [Google Scholar] [CrossRef] [PubMed]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Synthesis and characterization of chitosan-PVP-nanocellulose composites for in-vitro wound dressing application. Int. J. Biol. Macromol. 2017, 105, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tang, J.; Duan, Y.; Yu, A.; Berry, R.M.; Tam, K.C. Conductive cellulose nanocrystals with high cycling stability for supercapacitor applications. J. Mater. Chem. A 2014, 2, 19268–19274. [Google Scholar] [CrossRef]
- Going, R.J.; Sameoto, D.E.; Ayranci, C. Cellulose Nanocrystals: Dispersion in Co-Solvent Systems and Effects on Electrospun Polyvinylpyrrolidone Fiber Mats. J. Eng. Fiber. Fabr. 2015, 10, 155–163. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Kojima, Y.; Zhou, D. Preparation and Properties of Electrospun Poly(Vinyl Pyrrolidone)/Cellulose Nanocrystal/Silver Nanoparticle Composite Fibers. Materials 2016, 9, 523. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Tiwari, S.; Dharmalingam, K.; Shukla, I.; Pandey, L.M. Fabrication and characterization of chitosan, polyvinylpyrrolidone, and cellulose nanowhiskers nanocomposite films for wound healing drug delivery application. J. Biomed. Mater. Res. A 2017, 105, 2391–2404. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, Z. Iridescent Chiral Nematic Cellulose Nanocrystal/Polyvinylpyrrolidone Nanocomposite Films for Distinguishing Similar Organic Solvents. ACS Sustainable Chem. Eng. 2018, 6, 6192–6202. [Google Scholar] [CrossRef]
- Voronova, M.I.; Surov, O.V.; Zakharov, A.G. Nanocrystalline cellulose with various contents of sulfate groups. Carbohyd. Polym. 2013, 98, 465–469. [Google Scholar] [CrossRef]
- Surov, O.V.; Voronova, M.I.; Afineevskii, A.V.; Zakharov, A.G. Polyethylene oxide films reinforced by cellulose nanocrystals: Microstructure-properties relationship. Carbohyd. Polym. 2018, 181, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Boluk, Y.; Danumah, C. Analysis of cellulose nanocrystal rod lengths by dynamic light scattering and electron microscopy. J. Nanopart. Res. 2014, 16, 2174. [Google Scholar] [CrossRef]
- Fraschini, C.; Chauve, G.; Le Berre, J.-F.; Ellis, S.; Méthot, M.; O’Connor, B.; Bouchard, J. Critical discussion of light scattering and microscopy techniques for CNC particle sizing. Nord. Pulp Pap. Res. J. 2014, 29, 31–40. [Google Scholar] [CrossRef]
- Ioelovich, M. Characterization of various kinds of nanocellulose. In Handbook of Nanocellulose and Cellulose Nanocomposites, 1st ed.; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley: New York, NY, USA, 2017; Chapter 2; pp. 51–100. ISBN 978-3-527-33866-5. [Google Scholar]
- Zhou, C.; Chu, R.; Wu, R.; Wu, Q. Electrospun polyethylene Oxide/Cellulose nanocrystal composite nanofibrous mats with homogeneous and heterogeneous microstructures. Biomacromolecules 2011, 12, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, T.L.; Fel’dshtein, M.M.; Platé, N.A.; Kuptsov, S.A. Structure of stable H-bonded poly(N-vinylpyrrolidone)-water complexes. Polym. Sci. A 2000, 42, 989–1005. [Google Scholar]
- Lu, Y.; Armentrout, A.A.; Li, J.; Tekinalp, H.L.; Nanda, J.; Ozcan, S. A cellulose nanocrystal-based composite electrolyte with superior dimensional stability for alkaline fuel cell membranes. J. Mater. Chem. A 2015, 3, 13350–13356. [Google Scholar] [CrossRef]
- Niinivaara, E.; Faustini, M.; Tammelin, T.; Kontturi, E. Water Vapor Uptake of Ultrathin Films of Biologically Derived Nanocrystals: Quantitative Assessment with Quartz Crystal Microbalance and Spectroscopic Ellipsometry. Langmuir 2015, 31, 12170–12176. [Google Scholar] [CrossRef]
- Mathew, A.P.; Gong, G.; Bjorngrim, N.; Wixe, D.; Oksman, K. Moisture Adsorption Behavior and Its Impact on the Mechanical Properties of Cellulose Whiskers-Based Polyvinylacetate Nanocomposites. Polym. Eng. Sci. 2011, 51, 2136–2142. [Google Scholar] [CrossRef]
- Hakalahti, M.; Faustini, M.; Boissière, C.; Kontturi, E.; Tammelin, T. Interfacial Mechanisms of Water Vapor Sorption into Cellulose Nanofibril Films as Revealed by Quantitative Models. Biomacromolecules 2017, 18, 2951–2958. [Google Scholar] [CrossRef]
- Kontturi, E.; Tammelin, T.; Oesterberg, M. Cellulose—model films and the fundamental approach. Chem. Soc. Rev. 2006, 35, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, K.S.; Kontturi, E.; Laine, J. Specific water uptake of thin films from nanofibrillar cellulose. J. Mater. Chem. A 2013, 1, 13655–13663. [Google Scholar] [CrossRef]
- Hakalahti, M.; Mautner, A.; Johansson, L.-S.; Hänninen, T.; Setälä, H.; Kontturi, E.; Bismarck, A.; Tammelin, T. Direct Interfacial Modification of Nanocellulose Films for Thermoresponsive Membrane Templates. ACS Appl. Mater. Interfaces 2016, 8, 2923–2927. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, 1703779. [Google Scholar] [CrossRef] [PubMed]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Surface chemistry, morphological analysis and properties of cellulose nanocrystals with gradiented sulfation degrees. Nanoscale 2014, 10, 5384–5393. [Google Scholar] [CrossRef]
- Qin, X.; Xia, W.; Sinko, R.; Keten, S. Tuning Glass Transition in Polymer Nanocomposites with Functionalized Cellulose Nanocrystals through Nanoconfinement. Nano Lett. 2015, 15, 6738–6744. [Google Scholar] [CrossRef]
- Lizundia, E.; Fortunati, E.; Dominici, F.; Vilas, J.L.; León, L.M.; Armentano, I.; Torre, L.; Kenny, J.M. PLLA-grafted cellulose nanocrystals: Role of the CNC content and grafting on the PLA bionanocomposite film properties. Carbohyd. Polym. 2016, 142, 105–113. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids. In Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; ISBN 9781139878326. [Google Scholar]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.-L.; Heux, L.; Dubreuil, F.; Rochas, C. The Shape and Size Distribution of Crystalline Nanoparticles Prepared by Acid Hydrolysis of Native Cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal Structure and Hydrogen-Bonding System in Cellulose Iβ from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Grant, A.M.; Ma, R.; Zhang, S.; Tsukruk, V.V. Naturally-derived biopolymer nanocomposites: Interfacial design, properties and emerging applications. Mat. Sci. Eng. R 2018, 125, 1–41. [Google Scholar] [CrossRef]
- Rojas, O.J.; Lokanathan, A.L.; Kontturi, E.; Laine, J.; Bock, H. The unusual interactions between polymer grafted cellulose nanocrystal aggregates. Soft Matter 2013, 9, 8965–8973. [Google Scholar] [CrossRef] [Green Version]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid In. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. Modulation of Cellulose Nanocrystals Amphiphilic Properties to Stabilize Oil/Water Interface. Biomacromolecules 2012, 13, 267–275. [Google Scholar] [CrossRef]
- Munier, P.; Gordeyeva, K.; Bergström, L.; Fall, A.B. Directional Freezing of Nanocellulose Dispersions Aligns the Rod-Like Particles and Produces Low-Density and Robust Particle Networks. Biomacromolecules 2016, 17, 1875–1881. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing–thawing. J. Mater. Chem. A 2014, 2, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Hirai, H.; Yakura, N. Protecting Polymers in Suspensions of Metal Nanoparticles. Polym. Adv. Technol. 2001, 12, 724–733. [Google Scholar] [CrossRef]
- Sperling, L.H. Introduction to Physical Polymer Science, 4th ed.; John Wiley & Sons, Inc.: Bethlehem, PA, USA, 2006; ISBN 978-0-471-70606-9. [Google Scholar]
- Robinson, S.; Williams, P.A. Inhibition of Protein Adsorption onto Silica by Polyvinylpyrrolidone. Langmuir 2002, 18, 8743–8748. [Google Scholar] [CrossRef]
- Verma, S.; Huey, B.D.; Burgess, D.J. Scanning Probe Microscopy Method for Nanosuspension Stabilizer Selection. Langmuir 2009, 25, 12481–12487. [Google Scholar] [CrossRef] [PubMed]
- Kotel’nikova, N.E.; Panarin, E.F.; Kudina, N.P. Adsorption of polyvinylpyrrolidone from aqueous solutions on microcrystalline cellulose. Russ. J. Gen. Chem. 1997, 67, 315–319. [Google Scholar]
- Cheng, D.; Wen, Y.; Wang, L.; An, X.; Zhu, X.; Ni, Y. Adsorption of polyethylene glycol (PEG) onto cellulose nano-crystals to improve its dispersity. Carbohyd. Polym. 2015, 123, 157–163. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohyd. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Araki, J. Electrostatic or steric?—Preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter 2013, 9, 4125–4141. [Google Scholar] [CrossRef]














| Sample | Initial Mass Loss | First Degradation Stage | Second Degradation Stage | Total Mass Loss, % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ton, °C | Tmax, °C | Mass Loss, % | Ton, °C | Tmax, °C | Mass Loss, % | Ton, °C | Tmax, °C | Mass Loss, % | ||
| PVP | - | 76.4 | 14.6 | - | - | - | 405.4 | 432.3 | 80.9 | 95.5 |
| PVP/СNС-2.3 | - | 77.9 | 15.1 | - | - | - | 403.3 | 432.6 | 78.4 | 93.5 |
| PVP/СNС-6.7 | - | 80.6 | 13.7 | 240.1 | 264.5 | 3.9 | 402.9 | 433.8 | 76.6 | 94.2 |
| PVP/СNС-10.9 | - | 69.0 | 12.8 | 239.5 | 259.8 | 6.2 | 402.9 | 434.9 | 73.7 | 92.7 |
| PVP/СNС-19.6 | - | 72.8 | 12.2 | 232.6 | 252.1 | 11.7 | 403.3 | 436.6 | 64.0 | 87.9 |
| PVP/СNС-28.9 | - | 66.6 | 11.0 | 229.8 | 246.3 | 16.0 | 401.9 | 434.1 | 59.9 | 86.9 |
| PVP/СNС-37.9 | - | 64.8 | 10.6 | 226.4 | 242.4 | 19.6 | 398.2 | 430.4 | 53.8 | 84.0 |
| CNC | 63.0 | 82.2 | 3.0 | 154.5 | 169.8 | 37.8 | 334.1 | 376.4 | 30.1 | 70.9 |
| Sample | Tg, °C | |
|---|---|---|
| Heating | Cooling | |
| PVP | 180.2 | 166.7 |
| PVP/CNC-4.6 | 176.5 | 173.9 |
| PVP/CNC-10.6 | 178.3 | 174.7 |
| PVP/CNC-19.9 | 184.2 | 177.7 |
| PVP/CNC-28.9 | 198.5 | 186.4 |
| PVP/CNC-37.9 | 198.6 | 184.1 |
| Sample | Ultimate Tensile Strength (σmax), MPa | Elongation at Break (εb), % | Young’s Modulus (E), MPa |
|---|---|---|---|
| PVP | 6.6 | 5.5 | 120 |
| PVP/CNC-4.4 | 9.7 | 7.4 | 132 |
| PVP/CNC-8.4 | 17.2 | 4.2 | 413 |
| PVP/CNC-17.7 | 31.8 | 6.9 | 457 |
| PVP/CNC-26.8 | 38.4 | 3.0 | 1284 |
| PVP/CNC-29.2 | 43.8 | 2.5 | 1755 |
| PVP/CNC-39.9 | 25.3 | 2.1 | 1216 |
| Sample | Crystallographic Plane | ||
|---|---|---|---|
| (1–10) | (110) | (200) | |
| PVP/CNC-28.9 | 2.96 | 2.62 | 3.74 |
| PVP/CNC-37.9 | 2.15 | 2.53 | 4.19 |
| PVP/CNC-54.5 | 2.67 | 2.37 | 3.81 |
| PVP/CNC-70.6 | 2.48 | 2.42 | 3.74 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronova, M.; Rubleva, N.; Kochkina, N.; Afineevskii, A.; Zakharov, A.; Surov, O. Preparation and Characterization of Polyvinylpyrrolidone/Cellulose Nanocrystals Composites. Nanomaterials 2018, 8, 1011. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8121011
Voronova M, Rubleva N, Kochkina N, Afineevskii A, Zakharov A, Surov O. Preparation and Characterization of Polyvinylpyrrolidone/Cellulose Nanocrystals Composites. Nanomaterials. 2018; 8(12):1011. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8121011
Chicago/Turabian StyleVoronova, Marina, Natalia Rubleva, Nataliya Kochkina, Andrei Afineevskii, Anatoly Zakharov, and Oleg Surov. 2018. "Preparation and Characterization of Polyvinylpyrrolidone/Cellulose Nanocrystals Composites" Nanomaterials 8, no. 12: 1011. https://0-doi-org.brum.beds.ac.uk/10.3390/nano8121011