Carbon Nanotube-Graphene Hybrid Electrodes with Enhanced Thermo-Electrochemical Cell Properties
Abstract
:1. Introduction
2. Materials and Methods
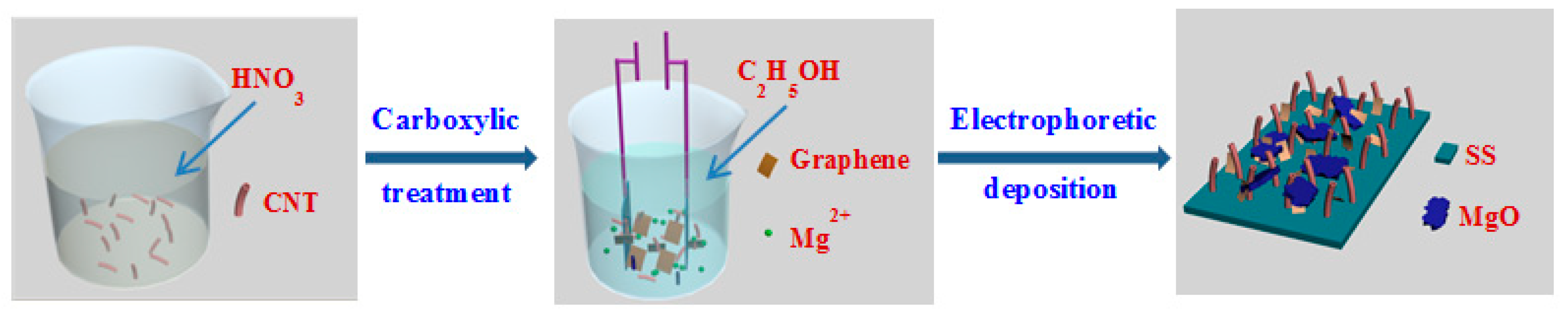
2.1. Synthesis of the CNT-Graphene Hybrid Electrodes
2.2. Characterization
2.3. TEC Measurements
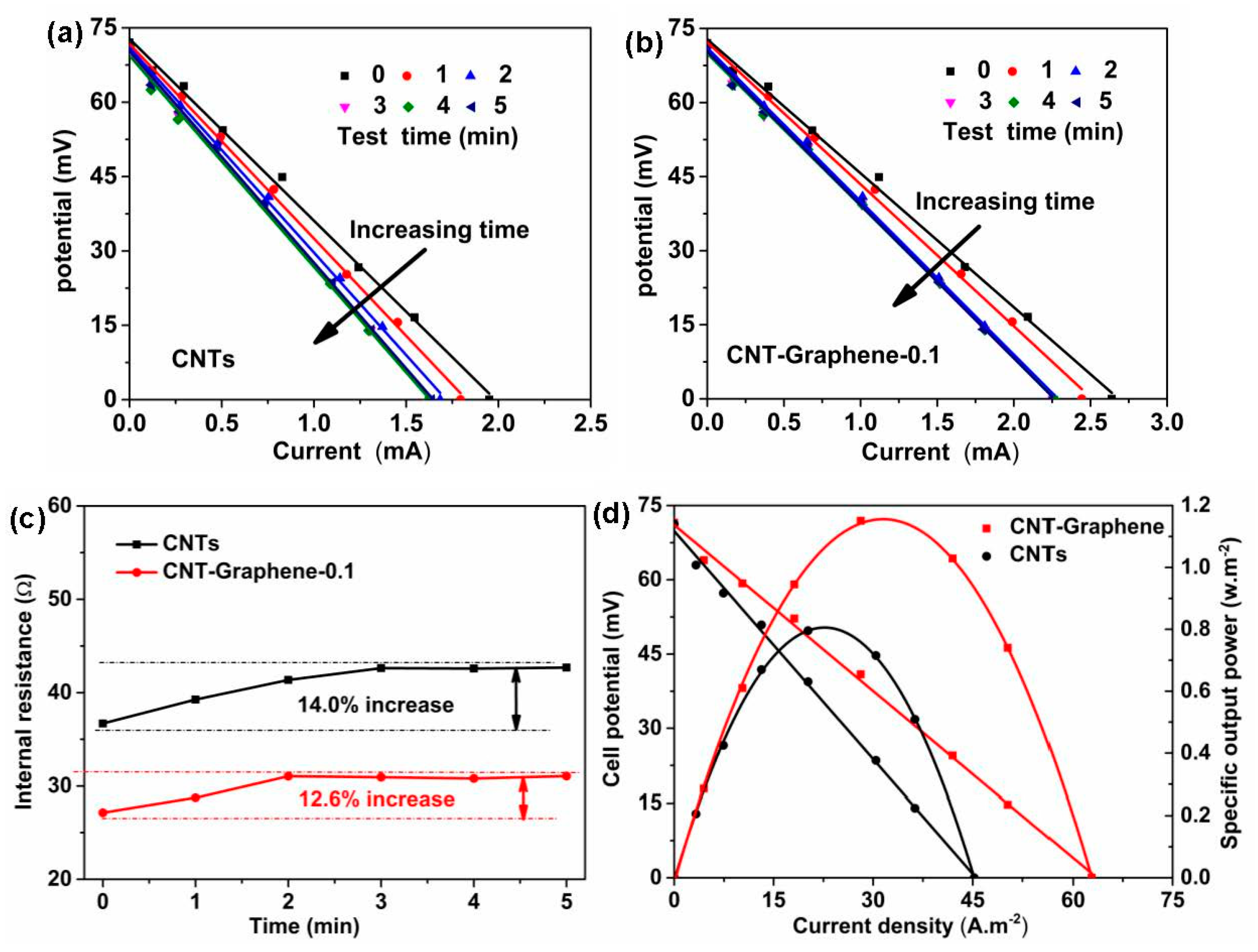
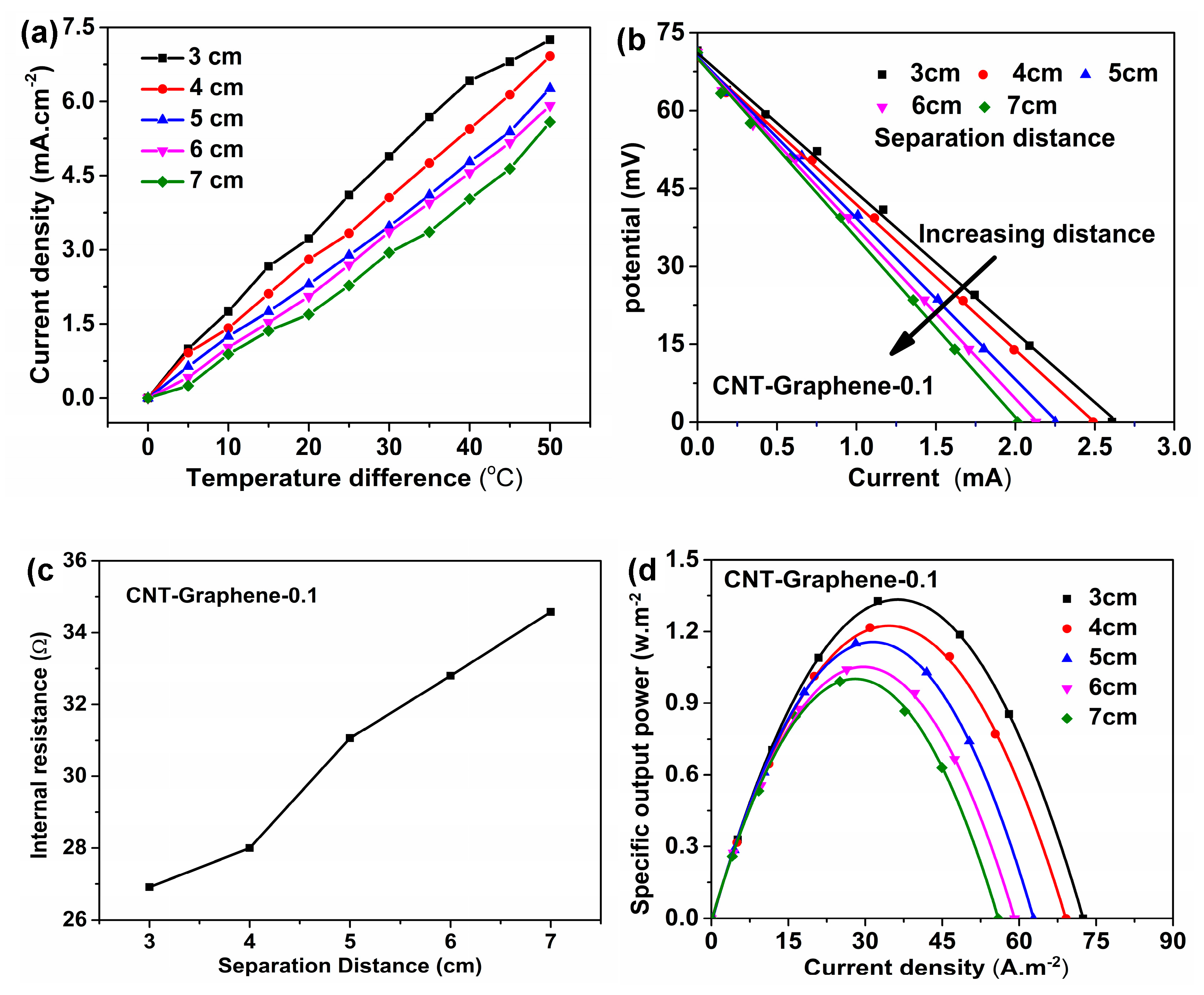
3. Results and Discussion
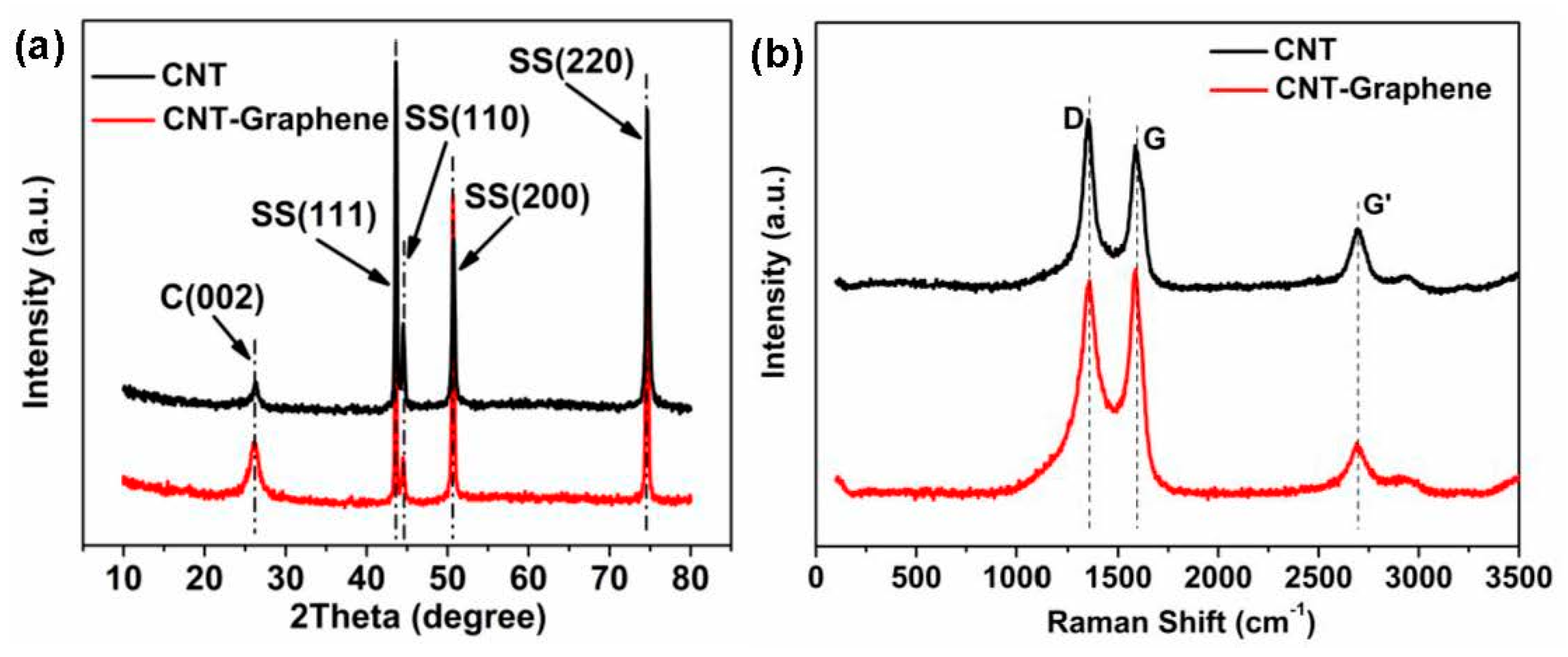
3.1. Structure Characterization
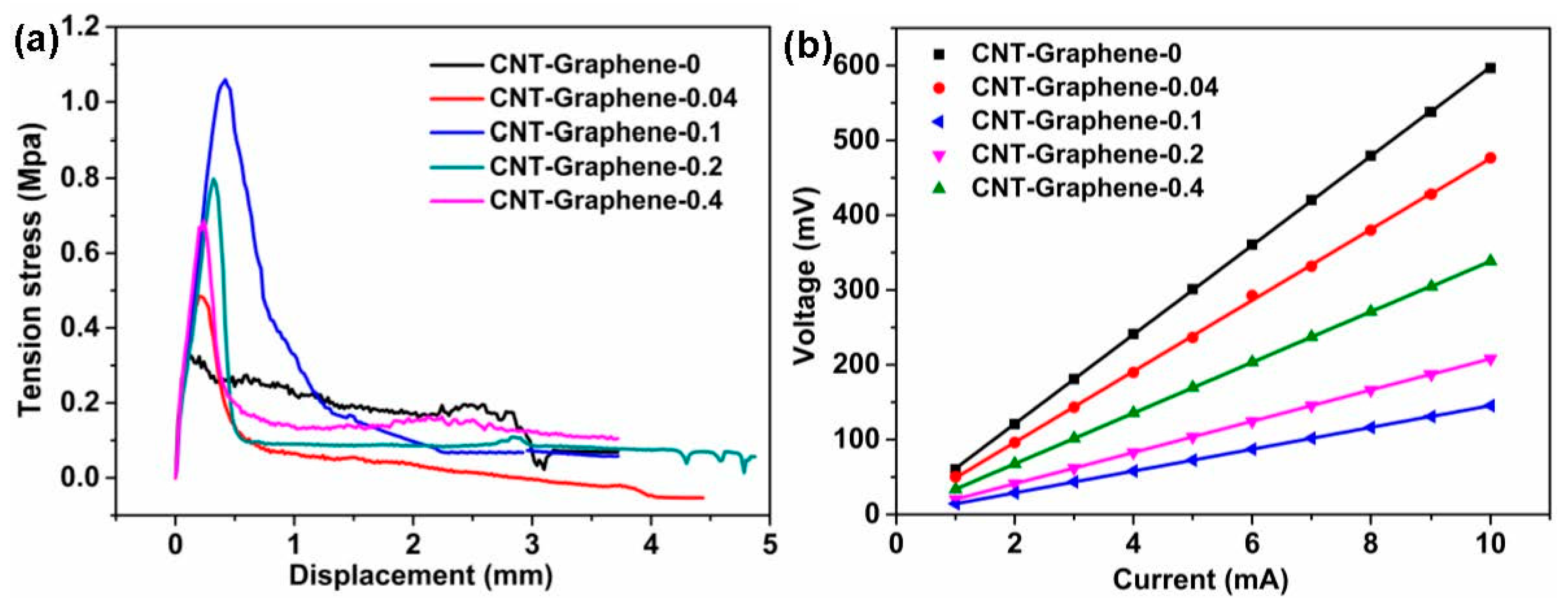
3.2. Tensile and the Surface Resistances Tests
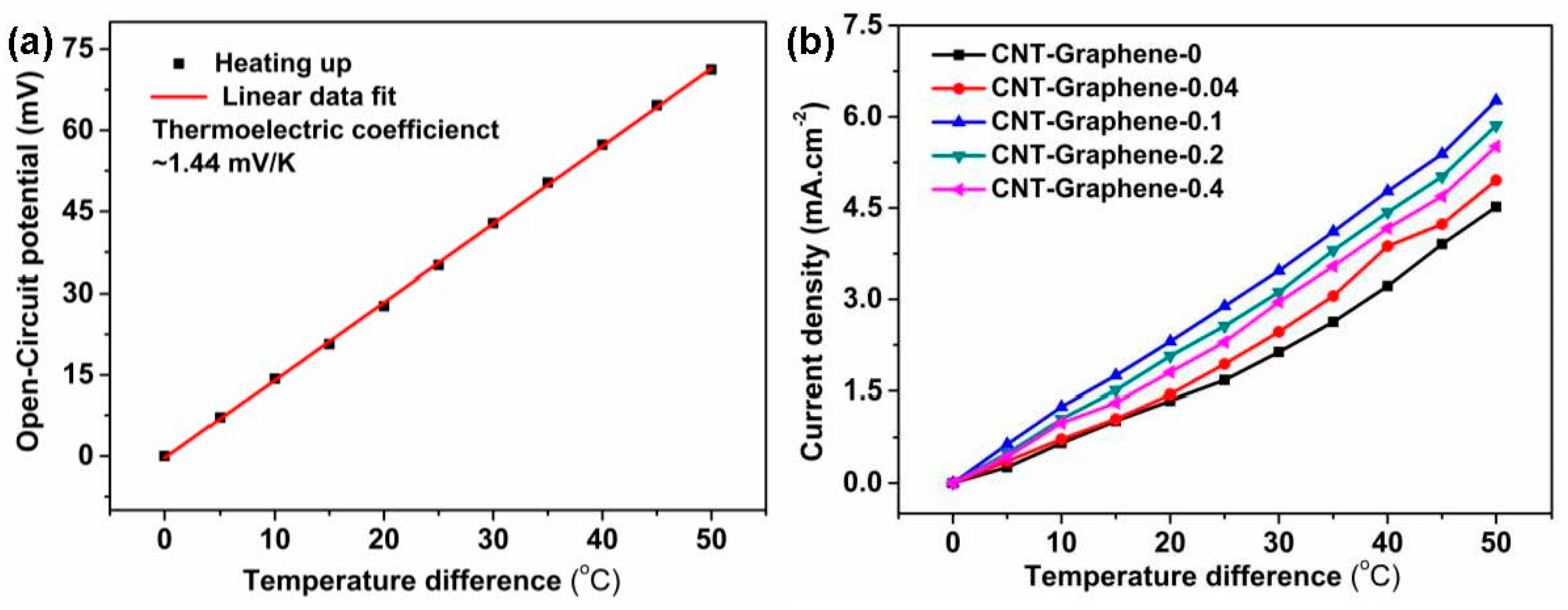
3.3. TEC Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Burmistrov, I.; Kovyneva, N.; Gorshkov, N.; Gorokhovsky, A.; Durakov, A.; Artyukhov, D.; Kiselev, N. Development of new electrode materials for thermo-electrochemical cells for waste heat harvesting. Renew. Energ. Focus 2019, 29, 42–48. [Google Scholar] [CrossRef]
- Alzahrani, H.A.H.; Black, J.J.; Goonetilleke, D.; Panchompoo, J.; Aldous, L. Combining thermogalvanic corrosion and thermogalvanic redox couples for improved electrochemical waste heat harvesting. Electrochem. Commun. 2015, 58, 76–79. [Google Scholar] [CrossRef]
- Kundu, A.; Fisher, T.S. Harnessing the thermogalvanic effect of the ferro/ferricyanide redox couple in a thermally chargeable supercapacitor. Electrochim. Acta 2018, 281, 357–369. [Google Scholar] [CrossRef]
- Hu, R.C.; Cola, B.A.; Haram, N.; Barisci, J.N.; Lee, S.; Stoughton, S.; Wallace, G.; Too, C.; Thomas, M.; Gestos, A.; et al. Harvesting waste thermal energy using a carbon-nanotube-based thermo-electrochemical cell. Nano Lett. 2010, 10, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.J.; Cao, M.X.; Xie, F.; Dong, C.K. Thermo-electrochemical cells based on carbon nanotube electrodes by electrophoretic deposition. Nano-Micro Lett. 2016, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, A.; Lin, C.H.; Buttry, D.A.; Mujica, V.; Taylor, R.A.; Prasher, R.S.; Phelan, P.E. Liquid thermoelectrics: Review of recent and limited new data of thermogalvanic cell experiments. Nanoscale Microscale Thermophys. Eng. 2013, 17, 304–323. [Google Scholar] [CrossRef]
- Salazar, P.F.; Kumar, S.; Cola, B.A. Nitrogen- and boron-doped carbon nanotube electrodes in a thermo-electrochemical cell. J. Electrochem. Soc. 2012, 159, B483–B488. [Google Scholar] [CrossRef]
- Im, H.; Moon, H.G.; Lee, J.S.; Chung, I.Y.; Kang, T.J.; Kim, Y.H. Flexible thermocells for utilization of body heat. Nano Res. 2014, 7, 443–452. [Google Scholar] [CrossRef]
- Yang, H.D.; Tufa, L.T.; Bae, K.M.; Kang, T.J. A tubing shaped, flexible thermal energy harvester based on a carbon nanotube sheet electrode. Carbon 2015, 86, 118–123. [Google Scholar] [CrossRef]
- Qian, W.J.; Li, M.J.; Chen, L.H.; Zhang, J.H.; Dong, C.K. Improving thermo-electrochemical cell performance by constructing Ag–MgO–CNTs nanocomposite electrodes. RSC Adv. 2015, 5, 97982–97987. [Google Scholar] [CrossRef]
- Zhao, F.; Qian, W.J.; Li, M.J.; Li, W.; Chen, L.H.; Zhong, F.Y.; Huang, W.J.; Dong, C.K. Directly grown carbon nanotube based hybrid electrodes with enhanced thermo-cell performances. RSC Adv. 2017, 7, 23890–23895. [Google Scholar] [CrossRef] [Green Version]
- Im, H.; Kim, T.; Song, H.; Choi, J.; Park, J.S.; Ovalle-Robles, R.; Yang, H.D.; Kihm, K.D.; Baughman, R.H.; Lee, H.H.; et al. High-efficiency electrochemical thermal energy harvester using carbon nanotube aerogel sheet electrodes. Nat. Commun. 2016, 7, 10600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, M.S.; Li, N.; Antiohos, D.; Razal, J.M.; Nattestad, A.; Beirne, S.; Fang, S.L.; Chen, Y.S.; Jalili, R.; Wallace, G.G.; et al. Carbon nanotube–reduced graphene oxide composites for thermal energy harvesting applications. Adv. Mater. 2013, 25, 6602–6606. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.J.; Fang, S.L.; Kozlov, M.E.; Haines, C.S.; Li, N.; Kim, Y.H.; Chen, Y.S.; Baughman, R.H. Electrical power from nanotube and graphene electrochemical thermal energy harvesters. Adv. Funct. Mater. 2012, 22, 477–489. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.C.; Minay, E.J.; Shaffer, M.S.P. Electrophoretic deposition of carbon nanotubes. Carbon 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Geng, X.M.; Zhang, Y.L.; Jiao, L.; Yang, L.; Hamel, J.; Giummarella, N.; Henriksson, G.; Zhang, L.M.; Zhu, H.L. Bioinspired ultrastable lignin cathode via graphene reconfiguration for energy storage. ACS Sustain. Chem. Eng. 2017, 5, 3553–3561. [Google Scholar] [CrossRef]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Cho, C.P. Mixed-phase MnO2/N-containing graphene composites applied as electrode active materials for flexible asymmetric solid-state supercapacitors. Nanomaterials 2018, 8, 924. [Google Scholar] [CrossRef]
- Wang, J.G.; Mu, X.J.; Sun, M.T. The Thermal, Electrical and Thermoelectric Properties of Graphene Nanomaterials. Nanomaterials 2019, 9, 218. [Google Scholar] [CrossRef]
- Ramkumar, R.; Minakshi, M. Fabrication of ultrathin CoMoO4 nanosheets modified with chitosan and their improved performance in energy storage device. Dalton Trans. 2015, 44, 6158–6168. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Sundaram, M.M. A biopolymer gel-decorated cobalt molybdate nanowafer: Effective graft polymer cross-linked with an organic acid for better energy storage. New J. Chem. 2016, 40, 2863–2877. [Google Scholar] [CrossRef]
- Ibrahim, R.; Hussein, M.Z.; Yusof, N.A.; Bakar, F.A. Carbon nanotube-quicklime nanocomposites prepared using a nickel catalyst supported on calcium oxide derived from carbonate stones. Nanomaterials 2019, 9, 1239. [Google Scholar] [CrossRef] [PubMed]
- Kholmanov, I.; Kim, J.; Ou, E.; Ruoff, R.S.; Shi, L. Continuous carbon nanotube–ultrathin graphite hybrid foams for increased thermal conductivity and suppressed subcooling in composite phase change materials. ACS Nano 2015, 9, 11699–11707. [Google Scholar] [CrossRef] [PubMed]
- Fagan, S.B.; Filho, A.G.S.; Lima, J.O.G.; Filho, J.M.; Ferreira, O.P.; Mazali, I.O.; Alves, O.L.; Dresselhaus, M.S. 1,2-Dichlorobenzene interacting with carbon nanotubes. Nano Lett. 2004, 4, 1285–1288. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Filho, A.G.S.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Cheng, H.H.; Dong, Z.L.; Hu, C.G.; Zhao, Y.; Hu, Y.; Qu, L.T.; Chen, N.; Dai, L.M. Textile electrodes woven by carbon nanotube–graphene hybrid fibers for flexible electrochemical capacitors. Nanoscale 2013, 5, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, L.; Zhang, C.G.; Casillas, G.; Sun, Z.; Yan, Z.; Ruan, G.; Peng, Z.; Raji, A.-R.O.; Kittrell, C.; et al. A seamless three-dimensional carbon nanotube graphene hybrid material. Nat. Commun. 2012, 3, 1225. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Kim, J. Thermal conductivity of a graphene oxide–carbon nanotube hybrid/epoxy composite. Carbon 2012, 50, 5429–5440. [Google Scholar] [CrossRef]
- Quickenden, T.I.; Mua, Y. A review of power generation in aqueous thermogalvanic cells. J. Electrochem. Soc. 1995, 142, 3985–3994. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal conductance of an individual single-wall carbon nanotube above room temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Berber, S.; Kwon, Y.-K.; Tomanek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613–4616. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, J.S.; Lee, G.; Yoon, H.; Yoon, J.; Kang, T.J.; Kim, Y.H. High thermopower of ferri/ferrocyanide redox couple in organic-water solutions. Nano Energy 2017, 31, 160–167. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Qian, W.; Huang, W.; Liu, B.; Lin, H.; Dong, C. Carbon Nanotube-Graphene Hybrid Electrodes with Enhanced Thermo-Electrochemical Cell Properties. Nanomaterials 2019, 9, 1450. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9101450
Zhou Y, Qian W, Huang W, Liu B, Lin H, Dong C. Carbon Nanotube-Graphene Hybrid Electrodes with Enhanced Thermo-Electrochemical Cell Properties. Nanomaterials. 2019; 9(10):1450. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9101450
Chicago/Turabian StyleZhou, Yuqing, Weijin Qian, Weijun Huang, Boyang Liu, Hao Lin, and Changkun Dong. 2019. "Carbon Nanotube-Graphene Hybrid Electrodes with Enhanced Thermo-Electrochemical Cell Properties" Nanomaterials 9, no. 10: 1450. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9101450



