Magnetic-Assisted Cell Alignment within a Magnetic Nanoparticle-Decorated Reduced Graphene Oxide/Collagen 3D Nanocomposite Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of M-rGO
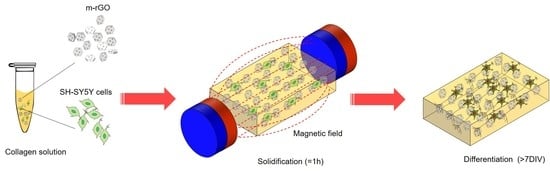
2.3. Preparation of Collagen and M-rGO/Collagen Composites
2.4. Cell Culture
2.5. Live/Dead Cell Assay
2.6. Immunofluorescence Staining
2.7. Measurement of Cell Orientation
2.8. Calcium Imaging
3. Results
3.1. Synthesis and Characterization of M-rGO
3.2. Biocompatibility of M-rGO/Collagen Scaffolds
3.3. Orientation of Cells within M-rGO/Collagen Scaffolds
3.4. Differentiation of SH-SY5Y Cells within M-rGO/Collagen Scaffolds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A.; Phillips, J.B. Cell Responses to Biomimetic Protein Scaffolds Used in Tissue Repair and Engineering. Int. Rev. Cytol. 2007, 262, 75–150. [Google Scholar] [PubMed]
- Deumens, R.; Bozkurt, A.; Meek, M.F.; Marcus, M.A.E.; Joosten, E.A.J.; Weis, J.; Brook, G.A. Repairing injured peripheral nerves: Bridging the gap. Prog. Neurobiol. 2010, 92, 245–276. [Google Scholar] [CrossRef] [PubMed]
- Leijten, J.; Seo, J.; Yue, K.; Trujillo-de Santiago, G.; Tamayol, A.; Ruiz-Esparza, G.U.; Shin, S.R.; Sharifi, R.; Noshadi, I.; Álvarez, M.M.; et al. Spatially and temporally controlled hydrogels for tissue engineering. Mater. Sci. Eng. R Rep. 2017, 119, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Cannizzaro, C.; Vunjak-Novakovic, G.; Langer, R.; Vacanti, C.A.; Farokhzad, O.C. Nanofabrication and Microfabrication of Functional Materials for Tissue Engineering. Tissue Eng. 2007, 13, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Yin, P.T.; Uehara, T.M.; Chueng, S.T.D.; Yang, L.; Lee, K.B. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv. Mater. 2014, 26, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Mullins, M.E.; Cregg, J.M.; Hurtado, A.; Oudega, M.; Trombley, M.T.; Gilbert, R.J. Creation of highly aligned electrospun Poly-L-lactic acid fibers for nerve regeneration applications. J. Neural Eng. 2009, 6, 016001. [Google Scholar] [CrossRef]
- Qing, H.; Jin, G.; Zhao, G.; Huang, G.; Ma, Y.; Zhang, X.; Sha, B.; Luo, Z.; Lu, T.J.; Xu, F. Heterostructured Silk-Nanofiber-Reduced Graphene Oxide Composite Scaffold for SH-SY5Y Cell Alignment and Differentiation. ACS Appl. Mater. Interfaces 2018, 10, 39228–39237. [Google Scholar] [CrossRef]
- Vader, D.; Kabla, A.; Weitz, D.; Mahadevan, L. Strain-induced alignment in collagen gels. PLoS ONE 2009, 4, e5902. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Borenstein, J.T. Biomaterials-based microfluidics for engineered tissue constructs. Soft Matter. 2010, 6, 4999–5015. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, J.; Qi, G.; He, C.; Wang, H. Anisotropic hydrogels fabricated with directional freezing and radiation-induced polymerization and crosslinking method. Mater. Lett. 2012, 89, 104–107. [Google Scholar] [CrossRef]
- Von Heimburg, D.; Zachariah, S.; Kühling, H.; Heschel, I.; Schoof, H.; Hafemann, B.; Pallua, N. Human preadipocytes seeded on freeze-dried collagen scaffolds investigated in vitro and in vivo. Biomaterials 2001, 22, 429–438. [Google Scholar] [CrossRef]
- Haque, M.A.; Kamita, G.; Kurokawa, T.; Tsujii, K.; Gong, J.P. Unidirectional alignment of lamellar bilayer in hydrogel: One-dimensional swelling, anisotropic modulus, and stress/strain tunable structural color. Adv. Mater. 2010, 22, 5110–5114. [Google Scholar] [CrossRef] [PubMed]
- Brandenberg, N.; Lutolf, M.P. In Situ Patterning of Microfluidic Networks in 3D Cell-Laden Hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gurkan, U.A.; Dehen, C.J.; Tate, M.P.; Hillhouse, H.W.; Simpson, G.J.; Akkus, O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials 2008, 29, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Kaufman, L.J. Flow and magnetic field induced collagen alignment. Biomaterials 2007, 28, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Im, S.K.; Oh, S.J.; Jeong, S.; Yoon, E.S.; Lee, C.J.; Choi, N.; Hur, E.M. Anisotropically organized three-dimensional culture platform for reconstruction of a hippocampal neural network. Nat. Commun. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, J.; Klueber, K.M.; Guo, Z.; Lu, C.; Winstead, W.I.; Qiu, M.; Roisen, F.J. Role of Transcription Factors in Motoneuron Differentiation of Adult Human Olfactory Neuroepithelial-Derived Progenitors. Stem Cells 2005, 24, 434–442. [Google Scholar] [CrossRef]
- Mothe, A.J.; Tam, R.Y.; Zahir, T.; Tator, C.H.; Shoichet, M.S. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials 2013, 34, 3775–3783. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Park, S.-J.; Choi, J.-W. Electrical Property of Graphene and Its Application to Electrochemical Biosensing. Nanomaterials 2019, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, T.; Choi, J.-W. Nano-Biosensor for Monitoring the Neural Differentiation of Stem Cells. Nanomaterials 2016, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Yoon, J.; Lee, Y.; Cho, H.-Y.; Lee, J.-Y.; Choi, J.-W. H2O2 sensor consisted of hemoglobin-DNA conjugate on nanoporous gold thin film electrode with electrochemical signal enhancement stem cell differentiation using a graphene-Au hybrid. Nano Converg. 2019, 6, 1. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chase, E.-J.; Park, S.-J.; Choi, J.-W. Label-free detection of γ-aminobytyric acid based on silicon nanowire biosensor. Nano Converg. 2019, 6, 5. [Google Scholar] [CrossRef]
- Dideikin, A.T.; Vul’, A.Y. Graphene Oxide and Derivatives: The Place in Graphene Family. Front. Phys. 2019, 6, 1–13. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J. Fast and simple method for screening of single-stranded DNA breaking photosensitizers using graphene oxide. Nano Converg. 2018, 5, 29. [Google Scholar] [CrossRef]
- Pan, H.; Low, S.; Weerasuriya, N.; Wang, B.; Shon, Y.S. Morphological transformation of gold nanoparticles on graphene oxide: Effects of capping ligands and surface interactions. Nano Converg. 2019, 6, 2. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamie, S.; Ghasemi, E. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon 2014, 66, 395–406. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, Y.; Liang, L.; Wei, M.; Hu, W.; Li, X.; Huang, Q. Effect of graphene oxide on undifferentiated and retinoic acid-differentiated SH-SY5Y cells line. Nanoscale 2012, 4, 3861–3866. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Staunton, J.R.; Tanner, K. Independent Control of Topography for 3D Patterning of the ECM Microenvironment. Adv. Mater. 2016, 28, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Antman-Passig, M.; Shefi, O. Remote Magnetic Orientation of 3D Collagen Hydrogels for Directed Neuronal Regeneration. Nano Lett. 2016, 16, 2567–2573. [Google Scholar] [CrossRef] [PubMed]
- Omidinia-Anarkoli, A.; Boesveld, S.; Tuvshindorj, U.; Rose, J.C.; Haraszti, T.; De Laporte, L. An Injectable Hybrid Hydrogel with Oriented Short Fibers Induces Unidirectional Growth of Functional Nerve Cells. Small 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Rose, J.C.; Cámara-Torres, M.; Rahimi, K.; Köhler, J.; Möller, M.; De Laporte, L. Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Le Ferrand, H.; Bolisetty, S.; Demirörs, A.F.; Libanori, R.; Studart, A.R.; Mezzenga, R. Magnetic assembly of transparent and conducting graphene-based functional composites. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 73, 1–6. [Google Scholar] [CrossRef]
- Tonazzini, I.; Cecchini, A.; Elgersma, Y.; Cecchini, M. Interaction of SH-SY5Y cells with nanogratings during neuronal differentiation: Comparison with primary neurons. Adv. Healthc. Mater. 2014, 3, 581–587. [Google Scholar] [CrossRef]
- Buttiglione, M.; Vitiello, F.; Sardella, E.; Petrone, L.; Nardulli, M.; Favia, P.; D’Agostino, R.; Gristina, R. Behaviour of SH-SY5Y neuroblastoma cell line grown in different media and on different chemically modified substrates. Biomaterials 2007, 28, 2932–2945. [Google Scholar] [CrossRef]
- Filograna, R.; Civiero, L.; Ferrari, V.; Codolo, G.; Greggio, E.; Bubacco, L.; Beltramini, M.; Bisaglia, M. Analysis of the catecholaminergic phenotype in human SH-SY5Y and BE (2)-M17 neuroblastoma cell lines upon differentiation. PLoS ONE 2015, 10, e0136769. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Deng, Y.; Zhang, J.; Han, H.; Zhao, M.; Li, Y.; Zhang, C.; Tian, J.; Bing, G.; Zhao, L. PINK1/Parkin-mediated mitophagy alleviates chlorpyrifos-induced apoptosis in SH-SY5Y cells. Toxicology 2015, 334, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisanic, T.R.; Blackwell, J.D.; Shubayev, V.I.; Fiñones, R.R.; Jin, S. Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 2007, 28, 2572–2581. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santhosh, M.; Choi, J.-H.; Choi, J.-W. Magnetic-Assisted Cell Alignment within a Magnetic Nanoparticle-Decorated Reduced Graphene Oxide/Collagen 3D Nanocomposite Hydrogel. Nanomaterials 2019, 9, 1293. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9091293
Santhosh M, Choi J-H, Choi J-W. Magnetic-Assisted Cell Alignment within a Magnetic Nanoparticle-Decorated Reduced Graphene Oxide/Collagen 3D Nanocomposite Hydrogel. Nanomaterials. 2019; 9(9):1293. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9091293
Chicago/Turabian StyleSanthosh, Mallesh, Jin-Ha Choi, and Jeong-Woo Choi. 2019. "Magnetic-Assisted Cell Alignment within a Magnetic Nanoparticle-Decorated Reduced Graphene Oxide/Collagen 3D Nanocomposite Hydrogel" Nanomaterials 9, no. 9: 1293. https://0-doi-org.brum.beds.ac.uk/10.3390/nano9091293