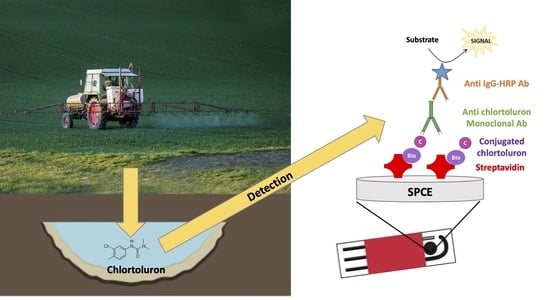
Monoclonal Antibody-Based Immunosensor for the Electrochemical Detection of Chlortoluron Herbicide in Groundwaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Groundwater Sample Preparation
2.3. Materials
2.4. Development of Chlortoluron Detection Tools
2.4.1. Colorimetric Immunoassay
2.4.2. Electrochemical Immunosensor
2.5. Data Processing for the Determination of the Method’s Sensitivity
3. Results and Discussion
3.1. Development of the Colorimetric Immunoassay
3.1.1. Optimization of Reagent Concentrations
3.1.2. Immunoassay Detection of Chlortoluron
3.1.3. Cross-Reactivity Study
3.2. Development of the Electrochemical Immunosensor
3.2.1. Electrochemical Characterization of TMB on SPCE
3.2.2. Optimization of Reagent Concentrations
3.2.3. Immunosensor Calibration
3.2.4. Detection of Chlortoluron in Groundwater Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, C.D.; van Beinum, W. Pesticide transport via sub-surface drains in Europe. Environ. Pollut. 2009, 157, 3314–3324. [Google Scholar] [CrossRef]
- Nsibande, S.A.; Forbes, P.B.C. Fluorescence detection of pesticides using quantum dot materials—A review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar] [CrossRef]
- Giuliano, S.; Alletto, L.; Deswarte, C.; Perdrieux, F.; Daydé, J.; Debaeke, P. Reducing herbicide use and leaching in agronomically performant maize-based cropping systems: An 8-year study. Sci. Total Environ. 2021, 788, 147695. [Google Scholar] [CrossRef] [PubMed]
- Reus, J.; Leendertse, P.; Bockstaller, C.; Fomsgaard, I.; Gutsche, V.; Lewis, K.; Nilsson, C.; Pussemier, L.; Trevisan, M.; Van Der Werf, H.; et al. Comparison and evaluation of eight pesticide environmental risk indicators developed in Europe and recommendations for future use. Agric. Ecosyst. Environ. 2002, 90, 177–187. [Google Scholar] [CrossRef]
- Liu, J. Phenylurea Herbicides, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 2, ISBN 9780123743671. [Google Scholar]
- Agbaogun, B.K.; Fischer, K. Adsorption of phenylurea herbicides by tropical soils. Environ. Monit. Assess. 2020, 192, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Arshad, M.; Springael, D.; Sorensen, S.R.; Bending, G.D.; Devers-lamrani, M.; Maqbool, Z.; Martin-laurent, F. Abiotic and biotic processes governing the fate of phenylurea herbicides in soils: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1947–1998. [Google Scholar] [CrossRef]
- Hong, M.; Ping, Z.; Jian, X. Testicular toxicity and mechanisms of chlorotoluron compounds in the mouse. Toxicol. Mech. Methods 2007, 17, 483–488. [Google Scholar] [CrossRef]
- Valiente Moro, C.; Bricheux, G.; Portelli, C.; Bohatier, J. Comparative effects of the herbicides chlortoluron and mesotrione on freshwater microalgae. Environ. Toxicol. Chem. 2012, 31, 778–786. [Google Scholar] [CrossRef]
- Baran, N.; Surdyk, N.; Auterives, C. Pesticides in groundwater at a national scale (France): Impact of regulations, molecular properties, uses, hydrogeology and climatic conditions. Sci. Total Environ. 2021, 791, 148137. [Google Scholar] [CrossRef]
- Bringer, A.; Thomas, H.; Prunier, G.; Dubillot, E.; Clérandeau, C.; Pageaud, M.; Cachot, J. Toxicity and risk assessment of six widely used pesticides on embryo-larval development of the Pacific oyster, Crassostrea gigas. Sci. Total Environ. 2021, 779, 146343. [Google Scholar] [CrossRef] [PubMed]
- Gimsing, A.L.; Agert, J.; Baran, N.; Boivin, A.; Ferrari, F.; Gibson, R.; Hammond, L.; Hegler, F.; Jones, R.L.; König, W.; et al. Conducting groundwater monitoring studies in Europe for pesticide active substances and their metabolites in the context of Regulation (EC) 1107/2009. J. Consum. Prot. Food Saf. 2019, 14, 1–93. [Google Scholar] [CrossRef] [Green Version]
- Pietrzak, D.; Kania, J.; Malina, G.; Kmiecik, E.; Wątor, K. Pesticides from the EU First and Second Watch Lists in the Water Environment. Clean-Soil Air Water 2019, 47, 1800376. [Google Scholar] [CrossRef]
- Da Silva Sousa, J.; Do Nascimento, H.O.; De Oliveira Gomes, H.; Do Nascimento, R.F. Pesticide residues in groundwater and surface water: Recent advances in solid-phase extraction and solid-phase microextraction sample preparation methods for multiclass analysis by gas chromatography-mass spectrometry. Microchem. J. 2021, 168, 106359. [Google Scholar] [CrossRef]
- Guo, L.; Liu, J.; Li, J.; Hao, L.; Liu, W.; Wang, C.; Wu, Q.; Wang, Z. A core-shell structured magnetic covalent organic framework as a magnetic solid-phase extraction adsorbent for phenylurea herbicides. J. Chromatogr. A 2021, 1651, 462301. [Google Scholar] [CrossRef]
- Li, Y.; George, J.E.; McCarty, C.L.; Wendelken, S.C. Compliance analysis of phenylurea and related compounds in drinking water by liquid chromatography/electrospray ionization/mass spectrometry coupled with solid-phase extraction. J. Chromatogr. A 2006, 1134, 170–176. [Google Scholar] [CrossRef]
- Mughari, A.R.; Vázquez, P.P.; Galera, M.M. Analysis of phenylurea and propanil herbicides by solid-phase microextraction and liquid chromatography combined with post-column photochemically induced fluorimetry derivatization and fluorescence detection. Anal. Chim. Acta 2007, 593, 157–163. [Google Scholar] [CrossRef]
- Lin, H.H.; Sung, Y.H.; Huang, S. Da Solid-phase microextraction coupled with high-performance liquid chromatography for the determination of phenylurea herbicides in aqueous samples. J. Chromatogr. A 2003, 1012, 57–66. [Google Scholar] [CrossRef]
- Samsidar, A.; Siddiquee, S.; Shaarani, S.M. A review of extraction, analytical and advanced methods for determination of pesticides in environment and foodstuffs. Trends Food Sci. Technol. 2018, 71, 188–201. [Google Scholar] [CrossRef]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent advances in biosensor technology for potential applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merz, D.; Geyer, M.; Moss, D.A.; Ache, H.J. Chlorophyll fluorescence biosensor for the detection of herbicides. Fresenius’ J. Anal. Chem. 1996, 354, 299–305. [Google Scholar] [CrossRef]
- Védrine, C.; Leclerc, J.C.; Durrieu, C.; Tran-Minh, C. Optical whole-cell biosensor using Chlorella vulgaris designed for monitoring herbicides. Biosens. Bioelectron. 2003, 18, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Peña-Vázquez, E.; Maneiro, E.; Pérez-Conde, C.; Moreno-Bondi, M.C.; Costas, E. Microalgae fiber optic biosensors for herbicide monitoring using sol-gel technology. Biosens. Bioelectron. 2009, 24, 3538–3543. [Google Scholar] [CrossRef]
- Giardi, M.T.; Scognamiglio, V.; Rea, G.; Rodio, G.; Antonacci, A.; Lambreva, M.; Pezzotti, G.; Johanningmeier, U. Optical biosensors for environmental monitoring based on computational and biotechnological tools for engineering the photosynthetic D1 protein of Chlamydomonas reinhardtii. Biosens. Bioelectron. 2009, 25, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Gouzy, M.F.; Keß, M.; Krämer, P.M. A SPR-based immunosensor for the detection of isoproturon. Biosens. Bioelectron. 2009, 24, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Sablok, K.; Bhalla, V.; Suri, C.R. A novel disposable electrochemical immunosensor for phenyl urea herbicide diuron. Biosens. Bioelectron. 2011, 26, 4209–4212. [Google Scholar] [CrossRef]
- Baskeyfield, D.E.H.; Davis, F.; Magan, N.; Tothill, I.E. A membrane-based immunosensor for the analysis of the herbicide isoproturon. Anal. Chim. Acta 2011, 699, 223–231. [Google Scholar] [CrossRef]
- Bhalla, V.; Sharma, P.; Pandey, S.K.; Suri, C.R. Sensors and Actuators B: Chemical Impedimetric label-free immunodetection of phenylurea class of herbicides. Sens. Actuators B Chem. 2012, 171, 1231–1237. [Google Scholar] [CrossRef]
- Katmeh, M.F.; Aherne, G.W.; Stevenson, D. Competitive enzyme-linked immunosorbent assay for the determination of the phenylurea herbicide chlortoluron in water and biological fluids. Analyst 1996, 121, 1699–1703. [Google Scholar] [CrossRef]
- Istamboulié, G.; Paniel, N.; Zara, L.; Granados, L.R.; Barthelmebs, L.; Noguer, T. Development of an impedimetric aptasensor for the determination of aflatoxin M1 in milk. Talanta 2016, 146, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Bettazzi, F.; Natale, A.R.; Torres, E.; Palchetti, I. Glyphosate determination by coupling an immuno-magnetic assay with electrochemical sensors. Sensors 2018, 18, 2965. [Google Scholar] [CrossRef] [Green Version]
- Marquez, L.A.; Dunford, H.B. Mechanism of the oxidation of 3,5,3′,5′-tetramethylbenzidine by myeloperoxidase determined by transient- and steady-state kinetics. Biochemistry 1997, 36, 9349–9355. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fernández, B.; Mercader, J.V.; Checa-Orrego, B.I.; De La Escosura-Muñiz, A.; Costa-García, A. A monoclonal antibody-based immunosensor for the electrochemical detection of imidacloprid pesticide. Analyst 2019, 144, 2936–2941. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Yean, C.Y.; Shueb, R.H. Screen printed carbon electrode based electrochemical immunosensor for the detection of dengue NS1 antigen. Diagnostics 2014, 4, 165–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vásquez, G.; Rey, A.; Rivera, C.; Iregui, C.; Orozco, J. Amperometric biosensor based on a single antibody of dual function for rapid detection of Streptococcus agalactiae. Biosens. Bioelectron. 2017, 87, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Martín-Fernández, B.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J.; Frutos-Cabanillas, G.; De-los-Santos-Álvarez, N.; López-Ruiz, B. Strongly structured DNA sequences as targets for genosensing: Sensing phase design and coupling to PCR amplification for a highly specific 33-mer gliadin DNA fragment. Biosens. Bioelectron. 2014, 60, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Amaya-González, S.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Sensitive gluten determination in gluten-free foods by an electrochemical Aptamer-based assay. Anal. Bioanal. Chem. 2015, 407, 6021–6029. [Google Scholar] [CrossRef] [PubMed] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surribas, A.; Barthelmebs, L.; Noguer, T. Monoclonal Antibody-Based Immunosensor for the Electrochemical Detection of Chlortoluron Herbicide in Groundwaters. Biosensors 2021, 11, 513. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11120513
Surribas A, Barthelmebs L, Noguer T. Monoclonal Antibody-Based Immunosensor for the Electrochemical Detection of Chlortoluron Herbicide in Groundwaters. Biosensors. 2021; 11(12):513. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11120513
Chicago/Turabian StyleSurribas, Anaïs, Lise Barthelmebs, and Thierry Noguer. 2021. "Monoclonal Antibody-Based Immunosensor for the Electrochemical Detection of Chlortoluron Herbicide in Groundwaters" Biosensors 11, no. 12: 513. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11120513