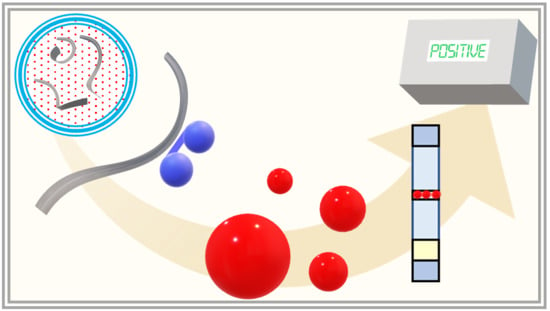
Gold–Oligonucleotide Nanoconstructs Engineered to Detect Conserved Enteroviral Nucleic Acid Sequences
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Gold Nanoparticles
2.2.2. Synthesis of Gold–Oligonucleotide Nanoconstructs
2.2.3. Dynamic Light Scattering (DLS)
2.2.4. Transmission Electron Microscopy
2.2.5. Zetasizer
2.2.6. Ultraviolet Spectrophotometry
2.2.7. Lateral Flow Assay
2.2.8. Cross-Sensitivity/ Selectivity Analyses
2.2.9. Electronic Desktop Detection
3. Results and Discussion
3.1. Design of Viral Sensitive Gold–Oligonucleotide Nanoconstructs
3.2. Synthesis and Characterisation of Gold–Oligonucleotide Nanoconstructs Sensitive to Conserved Target Enteroviral Nucleic Acid Sequences
3.3. Detecting Conserved Target Enteroviral Nucleic Acid Sequences with Gold–Oligonucleotide Nanoconstructs
3.4. Cross-Sensitivity/Selectivity Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baggen, J.; Thibaut, H.J.; Strating, J.; van Kuppeveld, F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018, 16, 368–381. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Li, T.; Jose, S.; Yip, C.C.Y.; Huang, Y.; Wong, E.Y.M.; Fan, R.Y.Y.; Cai, J.P.; Wernery, U.; et al. A novel dromedary camel enterovirus in the family Picornaviridae from dromedaries in the Middle East. J. Gen. Virol. 2015, 96, 1723–1731. [Google Scholar] [CrossRef]
- Du, J.; Lu, L.; Liu, F.; Su, H.X.; Dong, J.; Sun, L.L.; Zhu, Y.F.; Ren, X.W.; Yang, F.; Guo, F.; et al. Distribution and characteristics of rodent picornaviruses in China. Sci. Rep. 2016, 6, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haaheim, L.R.; Pattison, J.R.; Whitley, R.J. A Practical Guide to Clinical Virology; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Eccles, R. Understanding the symptoms of the common cold and influenza. Lancet Infect. Dis. 2005, 5, 718–725. [Google Scholar] [CrossRef]
- Noori, N.; Drake, J.M.; Rohani, P. Comparative epidemiology of poliovirus transmission. Sci. Rep. 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Shiley, K.T.; Lautenbach, E.; Lee, I. The Use of Antimicrobial Agents after Diagnosis of Viral Respiratory Tract Infections in Hospitalized Adults: Antibiotics or Anxiolytics? Infect. Control Hosp. Epidemiol. 2010, 31, 1177–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitney, C.G. Measuring progress on preventing pneumonia deaths: Are we there yet? Lancet Infect. Dis. 2017, 17, 1100–1101. [Google Scholar] [CrossRef] [Green Version]
- Gulliford, M.C.; Dregan, A.; Moore, M.V.; Ashworth, M.; van Staa, T.; McCann, G.; Charlton, J.; Yardley, L.; Little, P.; McDermott, L. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: Survey of 568 UK general practices. BMJ Open 2014, 4, e006245. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Chauhan, V.M.; Scurr, D.J.; Christie, T.; Telford, G.; Aylott, J.W.; Pritchard, D.I. The physicochemical fingerprint of Necator americanus. PLoS Negl. Trop. Dis. 2017, 11, e0005971. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.G.; Kim, Y.G.; Chung, B.G.; Demirci, U.; Khademhosseini, A. Nano/Microfluidics for diagnosis of infectious diseases in developing countries. Adv. Drug Deliv. Rev. 2010, 62, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Grigorieff, N.; Harrison, S.C. Near-atomic resolution reconstructions of icosahedral viruses from electron cryo-microscopy. Curr. Opin. Struct. Biol. 2011, 21, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Guo, W.; Yan, Y.Z.; Lee, S.; Wang, T. Label-free super-resolution imaging of adenoviruses by submerged microsphere optical nanoscopy. Light-Sci. Appl. 2013, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Hayden, R.T.; Gu, Z.; Ingersoll, J.; Abdul-Ali, D.; Shi, L.; Pounds, S.; Caliendo, A.M. Comparison of Droplet Digital PCR to Real-Time PCR for Quantitative Detection of Cytomegalovirus. J. Clin. Microbiol. 2013, 51, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From ELISA to next generation sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef] [PubMed]
- General Practice in the UK: British Medical Association. 2014. Available online: http://bma.org.uk/-/media/files/pdfs/news%20views%20analysis/press%20briefings/pressbriefinggeneralpracticeintheuk_july2014_v2.pdf (accessed on 6 August 2015).
- Clark, M.F.; Adams, A. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Peto, T.; Affron, D.; Afrough, B.; Agasu, A.; Ainsworth, M.; Allanson, A.; Allen, K.; Allen, C.; Archer, L.; Ashbridge, N.; et al. COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: A national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine 2021, 36, 100924. [Google Scholar] [CrossRef]
- Hang, V.T.; Nguyet, N.M.; Trung, D.T.; Tricou, V.; Yoksan, S.; Dung, N.M.; Van Ngoc, T.; Hien, T.T.; Farrar, J.; Wills, B.; et al. Diagnostic Accuracy of NS1 ELISA and Lateral Flow Rapid Tests for Dengue Sensitivity, Specificity and Relationship to Viraemia and Antibody Responses. PLoS Negl. Trop. Dis. 2009, 3, e360. [Google Scholar] [CrossRef] [PubMed]
- Chatziharalambous, D.; Lygirou, V.; Latosinska, A.; Stravodimos, K.; Vlahou, A.; Jankowski, V.; Zoidakis, J. Analytical Performance of ELISA Assays in Urine: One More Bottleneck towards Biomarker Validation and Clinical Implementation. PLoS ONE 2016, 11, e0149471. [Google Scholar] [CrossRef]
- Apilux, A.; Ukita, Y.; Chikae, M.; Chailapakul, O.; Takamura, Y. Development of automated paper-based devices for sequential multistep sandwich enzyme-linked immunosorbent assays using inkjet printing. Lab A Chip 2013, 13, 126–135. [Google Scholar] [CrossRef]
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020, 128, 104413. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.B.; Tang, T.H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.Y.; Lee, E.H.; Suh, A.Y.; Lee, S.J.; Lee, H. Oligonucleotide-based biosensors for in vitro diagnostics and environmental hazard detection. Anal. Bioanal. Chem. 2016, 408, 2383–2406. [Google Scholar] [CrossRef]
- Park, K.S. Nucleic acid aptamer-based methods for diagnosis of Infections. Biosens. Bioelectron. 2017, 102, 179–188. [Google Scholar] [CrossRef]
- Gonzalez, V.M.; Martin, M.E.; Fernandez, G.; Garcia-Sacristan, A. Use of Aptamers as Diagnostics Tools and Antiviral Agents for Human Viruses. Pharmaceuticals 2016, 9, 78. [Google Scholar] [CrossRef]
- Liu, J.W.; Cao, Z.H.; Lu, Y. Functional Nucleic Acid Sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [Green Version]
- Barthelmebs, L.; Jonca, J.; Hayat, A.; Prieto-Simon, B.; Marty, J.L. Enzyme-Linked Aptamer Assays (ELAAs), based on a competition format for a rapid and sensitive detection of Ochratoxin A in wine. Food Control 2011, 22, 737–743. [Google Scholar] [CrossRef]
- Huang, Y.K.; Chen, X.J.; Xia, Y.; Wu, S.J.; Duan, N.; Ma, X.Y.; Wang, Z.P. Selection, identification and application of a DNA aptamer against Staphylococcus aureus enterotoxin A. Anal. Methods 2014, 6, 690–697. [Google Scholar] [CrossRef]
- Yoshida, W.; Sode, K.; Ikebukuro, K. Homogeneous DNA sensing using enzyme-inhibiting DNA aptamers. Biochem. Biophys. Res. Commun. 2006, 348, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.R.; Yang, Y.X.; He, R.Y.; Park, Y.I.; Lee, A.; Bai, D.; Li, F.; Lu, T.J.; Xu, F.; Lin, M. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens. Actuators B Chem. 2018, 276, 48–56. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, J.; Luo, F.L.; Mohammed, A.B.; Zhang, X.L. Aptamer from whole-bacterium SELEX as new therapeutic reagent against virulent Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 2007, 357, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yigit, M.V.; Mazumdar, D.; Lu, Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.W.; Lee, J.H.; Lu, Y. Quantum dot encoding of aptamer-linked nanostructures for one-pot simultaneous detection of multiple analytes. Anal. Chem. 2007, 79, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.A.; Wang, J.; Kawde, A.N.; Xiang, Y.; Gothelf, K.V.; Collins, G. Quantum-dot/aptamer-based ultrasensitive multi-analyte electrochemical biosensor. J. Am. Chem. Soc. 2006, 128, 2228–2229. [Google Scholar] [CrossRef]
- Yigit, M.V.; Mazumdar, D.; Lu, Y. MRI detection of thrombin with aptamer functionalized superparamagnetic iron oxide nanoparticles. Bioconjugate Chem. 2008, 19, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Giamberardino, A.; Labib, M.; Hassan, E.M.; Tetro, J.A.; Springthorpe, S.; Sattar, S.A.; Berezovski, M.V.; DeRosa, M.C. Ultrasensitive Norovirus Detection Using DNA Aptasensor Technology. PLoS ONE 2013, 8, e0079087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Gao, X.; Liu, D.B.; Chen, X.Y. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.M.; Sun, M.; Lin, Y.; Liu, Y.J.; Luo, F.; Guo, L.H.; Qiu, B.; Lin, Z.Y.; Chen, G.N. Progress of Visual Biosensor Based on Gold Nanoparticles. Chin. J. Anal. Chem. 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Soh, J.H.; Lin, Y.Y.; Rana, S.; Ying, J.Y.; Stevens, M.M. Colorimetric Detection of Small Molecules in Complex Matrixes via Target-Mediated Growth of Aptamer-Functionalized Gold Nanoparticles. Anal. Chem. 2015, 87, 7644–7652. [Google Scholar] [CrossRef]
- Zhou, W.L.; Kong, W.J.; Dou, X.W.; Zhao, M.; Ouyang, Z.; Yang, M.H. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1022, 102–108. [Google Scholar] [CrossRef]
- Liu, J.; Mazumdar, D.; Lu, Y. A simple and sensitive “dipstick” test in serum based on lateral flow separation of aptamer-linked nanostructures. Angew. Chem. Int. Ed. 2006, 45, 7955–7959. [Google Scholar] [CrossRef]
- Pavlov, V.; Xiao, Y.; Shlyahovsky, B.; Willner, I. Aptamer-functionalized Au nanoparticles for the amplified optical detection of thrombin. J. Am. Chem. Soc. 2004, 126, 11768–11769. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.H.; Liu, X.F.; Liang, Z.Q.; Song, S.P.; Li, W.X.; Li, G.X.; Fan, C.H. A gold nanoparticle-based aptamer target binding readout for ATP assay. Adv. Mater. 2007, 19, 3943–3946. [Google Scholar] [CrossRef]
- Huang, C.C.; Huang, Y.F.; Cao, Z.H.; Tan, W.H.; Chang, H.T. Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal. Chem. 2005, 77, 5735–5741. [Google Scholar] [CrossRef] [PubMed]
- Wandtke, T.; Wozniak, J.; Kopinski, P. Aptamers in Diagnostics and Treatment of Viral Infections. Viruses 2015, 7, 751–780. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: http://amr-review.org/ (accessed on 6 August 2015).
- Liu, J.; Lu, Y. Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat. Protoc. 2006, 1, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kafasla, P.; Morgner, N.; Robinson, C.V.; Jackson, R.J. Polypyrimidine tract-binding protein stimulates the poliovirus IRES by modulating eIF4G binding. EMBO J. 2010, 29, 3710–3722. [Google Scholar] [CrossRef] [Green Version]
- Malnou Cécile, E.; Pöyry Tuija, A.A.; Jackson Richard, J.; Kean Katherine, M. Poliovirus Internal Ribosome Entry Segment Structure Alterations That Specifically Affect Function in Neuronal Cells: Molecular Genetic Analysis. J. Virol. 2002, 76, 10617–10626. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.X.; Carratala, A.; Shim, H.; Bachmann, V.; Jensen, J.D.; Kohn, T. Resistance of Echovirus 11 to ClO2 Is Associated with Enhanced Host Receptor Use, Altered Entry Routes, and High Fitness. Environ. Sci. Technol. 2017, 51, 10746–10755. [Google Scholar] [CrossRef] [Green Version]
- Torrey, J.; von Gunten, U.; Kohn, T. Differences in Viral Disinfection Mechanisms as Revealed by Quantitative Transfection of Echovirus 11 Genomes. Appl. Environ. Microbiol. 2019, 85, 14. [Google Scholar] [CrossRef] [Green Version]
- Scherer, S.E.; Muzny, D.M.; Buhay, C.J.; Chen, R.; Cree, A.; Ding, Y.; Dugan-Rocha, S.; Gill, R.; Gunaratne, P.; Harris, R.A.; et al. The finished DNA sequence of human chromosome 12. Nature 2006, 440, 346–351. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.Q.; Dever, B.; Li, X.F.; Le, X.C. Thermal Stability of DNA Functionalized Gold Nanoparticles. Bioconjugate Chem. 2013, 24, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Swart, M.; Guerra, C.F.; Bickelhaupt, F.M. Hydrogen bonds of RNA are stronger than those of DNA, but NMR monitors only presence of methyl substituent in uracil/thymine. J. Am. Chem. Soc. 2004, 126, 16718–16719. [Google Scholar] [CrossRef] [PubMed]
- Wald, G. Human vision and the spectrum. Science 1945, 101, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Wilchek, M.; Bayer, E.A. The Avidin Biotin Complex in Bioanalytical Applications. Anal. Biochem. 1988, 171, 1–32. [Google Scholar] [CrossRef]
- Draz, M.S.; Shafiee, H. Applications of gold nanoparticles in virus detection. Theranostics 2018, 8, 1985–2017. [Google Scholar] [CrossRef] [PubMed]
- Peltola, V.; Waris, M.; Kainulainen, L.; Kero, J.; Ruuskanen, O. Virus shedding after human rhinovirus infection in children, adults and patients with hypogammaglobulinaemia. Clin. Microbiol. Infect. 2013, 19, E322–E327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granados, A.; Goodall, E.C.; Luinstra, K.; Smieja, M.; Mahony, J. Comparison of asymptomatic and symptomatic rhinovirus infections in university students: Incidence, species diversity, and viral load. Diagn. Microbiol. Infect. Dis. 2015, 82, 292–296. [Google Scholar] [CrossRef]
- Harvala, H.; Broberg, E.; Benschop, K.; Berginc, N.; Ladhani, S.; Susi, P.; Christiansen, C.; McKenna, J.; Allen, D.; Makiello, P.; et al. Recommendations for enterovirus diagnostics and characterisation within and beyond Europe. J. Clin. Virol. 2018, 101, 11–17. [Google Scholar] [CrossRef]
- Timm, A.; Yin, J. Kinetics of virus production from single cells. Virology 2012, 424, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, T.; Ishii, A.; Segawa, T.; Takagi, Y.; Kobayashi, Y.; Itou, T. Establishing conditions for the storage and elution of rabies virus RNA using FTA (R) cards. J. Vet. Med. Sci. 2015, 77, 461–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, P.; Kumar, B.K.; Deekshit, V.K.; Karunasagar, I.; Karunasagar, I. Detection technologies and recent developments in the diagnosis of COVID-19 infection. Appl. Microbiol. Biotechnol. 2021, 105, 441–455. [Google Scholar] [CrossRef]
- Wang, R.; Hozumi, Y.; Yin, C.; Wei, G.-W. Mutations on COVID-19 diagnostic targets. Genomics 2020, 112, 5204–5213. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D. Genetic mechanisms of critical illness in Covid-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S. Remdesivir for the treatment of Covid-19. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauhan, V.M.; Elsutohy, M.M.; McClure, C.P.; Irving, W.L.; Roddis, N.; Aylott, J.W. Gold–Oligonucleotide Nanoconstructs Engineered to Detect Conserved Enteroviral Nucleic Acid Sequences. Biosensors 2021, 11, 238. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11070238
Chauhan VM, Elsutohy MM, McClure CP, Irving WL, Roddis N, Aylott JW. Gold–Oligonucleotide Nanoconstructs Engineered to Detect Conserved Enteroviral Nucleic Acid Sequences. Biosensors. 2021; 11(7):238. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11070238
Chicago/Turabian StyleChauhan, Veeren M., Mohamed M. Elsutohy, C. Patrick McClure, William L. Irving, Neil Roddis, and Jonathan W. Aylott. 2021. "Gold–Oligonucleotide Nanoconstructs Engineered to Detect Conserved Enteroviral Nucleic Acid Sequences" Biosensors 11, no. 7: 238. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11070238