DNA Aptamer Functionalized Hydrogels for Interferometric Fiber-Optic Based Continuous Monitoring of Potassium Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sensor Fabrication
2.3. Determination of Biosensor Swelling Response
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viera, A.J.; Wouk, N. Potassium Disorders: Hypokalemia and Hyperkalemia. Am. Fam. Physician 2015, 92, 487–495. [Google Scholar] [PubMed]
- Ferreira, J.P.; Butler, J.; Rossignol, P.; Pitt, B.; Anker, S.D.; Kosiborod, M.; Lund, L.H.; Bakris, G.L.; Weir, M.R.; Zannad, F. Abnormalities of Potassium in Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 2836–2850. [Google Scholar] [CrossRef] [PubMed]
- Tongyoo, S.; Viarasilpa, T.; Permpikul, C. Serum potassium levels and outcomes in critically ill patients in the medical intensive care unit. J. Int. Med. Res. 2018, 46, 1254–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, A.; O’Donnell, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Ah, S.T.L.; Wei, L.; Diaz, R.; et al. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: A community-level prospective epidemiological cohort study. Lancet 2018, 392, 496–506. [Google Scholar] [CrossRef]
- Kardalas, E.; Paschou, S.A.; Anagnostis, P.; Muscogiuri, G.; Siasos, G.; Vryonidou, A. Hypokalemia: A clinical update. Endocr. Connect. 2018, 7, R135–R146. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.D.; Btaiche, I.F.; Sacks, G.S.; Kudsk, K.A. Treatment of electrolyte disorders in adult patients in the intensive care unit. Am. J. Health Syst. Pharm. 2005, 62, 1663–1682. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.A.; King, J.; Kathe, N.; Erbach, K.; Stojakovic, J.; Tran, J.; Clem, O.A. Effectiveness and Safety of Potassium Replacement in Critically Ill Patients: A Retrospective Cohort Study. Crit. Care Nurse 2019, 39, E13–E18. [Google Scholar] [CrossRef] [Green Version]
- Brueske, B.; Sidhu, M.S.; Schulman-Marcus, J.; Kashani, K.B.; Barsness, G.W.; Jentzer, J.C. Hyperkalemia Is Associated with Increased Mortality Among Unselected Cardiac Intensive Care Unit Patients. J. Am. Heart Assoc. 2019, 8, e011814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.-A.J.; Shahabi, S.; Mostafapour, E.; Purfakharan, M.; Fereshtehnejad, S.-M.; Amini, J.; Khojandi, M.; Raji, H. Comparison of the Serum Electrolyte Levels among Patients Died and Survived in the Intensive Care Unit. Tanaffos 2012, 11, 36–42. [Google Scholar]
- Hoppe, L.K.; Muhlack, D.C.; Koenig, W.; Carr, P.R.; Brenner, H.; Schoettker, B. Association of Abnormal Serum Potassium Levels with Arrhythmias and Cardiovascular Mortality: A Systematic Review and Meta-Analysis of Observational Studies. Cardiovasc. Drugs Ther. 2018, 32, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ Br. Med. J. 2013, 346, f1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keskin, M.; Kaya, A.; Tatlisu, M.A.; Hayiroglu, M.I.; Uzman, O.; Borklu, E.B.; Cinier, G.; Cakilli, Y.; Yaylak, B.; Eren, M. The effect of serum potassium level on in-hospital and long-term mortality in ST elevation myocardial infarction. Int. J. Cardiol. 2016, 221, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip 2004, 4, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Tejavibulya, N.; Colburn, D.A.M.; Marcogliese, F.A.; Yang, K.-A.; Guo, V.; Chowdhury, S.; Stojanovic, M.N.; Sia, S.K. Hydrogel Microfilaments toward Intradermal Health Monitoring. Iscience 2019, 21, 328–340. [Google Scholar] [CrossRef]
- Schwartz, I.L.; Thaysen, J.H. Excretion of sodium and potassium in human sweat. J. Clin. Investig. 1956, 35, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pant, V.; Tumbapo, A.; Karki, B. Inter-instrumental comparison for the measurement of electrolytes in patients admitted to the intensive care unit. Int. J. Gen. Med. 2017, 10, 145–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Triplett, K.E.; Wibrow, B.A.; Norman, R.; Hince, D.A.; Hardy, L.E.; Tan, S.; Ho, K.M.; Anstey, M.H. Can the blood gas analyser results be believed? A prospective multicentre study comparing haemoglobin, sodium and potassium measurements by blood gas analysers and laboratory auto-analysers. Anaesth. Intensive Care 2019, 47, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.W.; Covington, A.K.; Fogh-Andersen, N.; Külpmann, W.R.; Lewenstam, A.; Maas, A.H.J.; Müller-Plathe, O.; Sachs, C.; Siggaard-Andersen, O.; VanKessel, A.L.; et al. Recommendations for Measurement of and Conventions for Reporting Sodium and Potassium by Ion-Selective Electrodes in Undiluted Serum, Plasma or Whole Blood. Clin. Chem. Lab. Med. 2000, 38, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Uysal, H.B.; Avcil, M.; Yilmaz, M.; Dagh, B.; Bakis, M.; Omurlu, I.K. Comparison of different methods for measurement of electrolytes in patients admitted to the intensive care unit. Saudi Med. J. 2016, 37, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, E.; De Deco, P.; Trevisan, M.; Bellocco, R.; Lindholm, B.; Lund, L.H.; Coresh, J.; Carrero, J.J. A real-world cohort study on the quality of potassium and creatinine monitoring during initiation of mineralocorticoid receptor antagonists in patients with heart failure. Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Raebel, M.A.; McClure, D.L.; Simon, S.R.; Chan, K.A.; Feldstein, A.; Andrade, S.E.; Lafata, J.E.; Roblin, D.; Davis, R.L.; Gunter, M.J.; et al. Laboratory monitoring of potassium and creatinine in ambulatory patients receiving angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Pharmacoepidemiol. Drug Saf. 2007, 16, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Park, J.Y.; Kwon, O.S.; Joo, S.; Lee, C.-S.; Bae, J. Incorporation of hydrogel as a sensing medium for recycle of sensing material in chemical sensors. Appl. Surf. Sci. 2018, 429, 258–263. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tierney, S.; Falch, B.M.H.; Hjelme, D.R.; Stokke, B.T. Determination of Glucose Levels Using a Functionalized Hydrogel-Optical Fiber Biosensor: Toward Continuous Monitoring of Blood Glucose in Vivo. Anal. Chem. 2009, 81, 3630–3636. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.-H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 2000, 404, 588–590. [Google Scholar] [CrossRef]
- Chan, G.; Mooney, D.J. New materials for tissue engineering: Towards greater control over the biological response. Trends Biotechnol. 2008, 26, 382–392. [Google Scholar] [CrossRef]
- Bysell, H.; Malmsten, M. Visualizing the interaction between poly-L-lysine and poly( acrylic acid) microgels using microscopy techniques: Effect of electrostatics and peptide size. Langmuir 2006, 22, 5476–5484. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Raston, N.H.A.; Gu, M.B. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.H.; Saran, R.; Liu, J.W. Metal Sensing by DNA. Chem. Rev. 2017, 117, 8272–8325. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Jiao, X.; Liu, S.; Hao, M.; Cheng, S.; Zhang, P.; Wen, Y. Functional DNA-based hydrogel intelligent materials for biomedical applications. J. Mater. Chem. B 2020, 8, 1991–2009. [Google Scholar] [CrossRef] [PubMed]
- Gačanin, J.; Synatschke, C.V.; Weil, T. Biomedical Applications of DNA-Based Hydrogels. Adv. Funct. Mater. 2020, 30, 1906253. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2016, 16, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonasova, E.P.; Stokke, B.T. Bioresponsive DNA-co-polymer hydrogels for fabrication of sensors. Curr. Opin. Colloid Interface Sci. 2016, 26, 1–8. [Google Scholar] [CrossRef]
- Li, F.; Lyu, D.; Liu, S.; Guo, W. DNA Hydrogels and Microgels for Biosensing and Biomedical Applications. Adv. Mater. 2020, 32, 1806538. [Google Scholar] [CrossRef]
- Li, X.; Bai, T.; Li, Z.; Liu, L. Influence of the temperature on the hyper-elastic mechanical behavior of carbon black filled natural rubbers (vol 95, pg 136, 2016). Mech. Mater. 2016, 99, 136–145. [Google Scholar] [CrossRef]
- Liu, J.W. Oligonucleotide-functionalized hydrogels as stimuli responsive materials and biosensors. Soft Matter 2011, 7, 6757–6767. [Google Scholar] [CrossRef] [Green Version]
- Tierney, S.; Stokke, B.T. Development of an Oligonucleotide Functionalized Hydrogel Integrated on a High-Resolution Interferometric Readout Platform as a Label-Free Macromolecule Sensing Device. Biomacromolecules 2009, 10, 1619–1626. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Li, J.; Zhao, J.; Fan, C. Aptamer-based biosensors. Trac-Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Qin, H.; Ren, J.; Wang, J.; Luedtke, N.W.; Wang, E. G-Quadruplex-Modulated Fluorescence Detection of Potassium in the Presence of a 3500-Fold Excess of Sodium Ions. Anal. Chem. 2010, 82, 8356–8360. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Q.; Xiang, J.; Wang, L.; Zhang, X.; Lan, L.; Xu, S.; Yang, F.; Tang, Y. Novel fluorescent cationic benzothiazole dye that responds to G-quadruplex aptamer as a novel K+ sensor. Analyst 2017, 142, 3352–3355. [Google Scholar] [CrossRef] [PubMed]
- Schuewer, N.; Klok, H.-A. A Potassium-Selective Quartz Crystal Microbalance Sensor Based on Crown-Ether Functionalized Polymer Brushes. Adv. Mater. 2010, 22, 3251. [Google Scholar] [CrossRef]
- Zhang, Z.; Dou, Q.; Gao, H.; Bai, B.; Zhang, Y.; Hu, D.; Yetisen, A.K.; Butt, H.; Yang, X.; Li, C.; et al. 30 s Response Time of K+ Ion-Selective Hydrogels Functionalized with 18-Crown-6 Ether Based on QCM Sensor. Adv. Healthc. Mater. 2018, 7, 1700873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumbhat, S.; Singh, U. A potassium-selective electrochemical sensor based on crown-ether functionalized self assembled monolayer. J. Electroanal. Chem. 2018, 809, 31–35. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.; Islam, M.S.; Rahman, M.A.; Walsh, K.B.; Koley, G. Graphene field effect transistors for highly sensitive and selective detection of K+ ions. Sens. Actuators B Chem. 2017, 253, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Soda, Y.; Citterio, D.; Bakker, E. Equipment-Free Detection of K+ on Microfluidic Paper-Based Analytical Devices Based on Exhaustive Replacement with Ionic Dye in Ion-selective Capillary Sensors. ACS Sens. 2019, 4, 670–677. [Google Scholar] [CrossRef]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2015–2019). Anal. Chem. 2020, 92, 397–430. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Kumar, R. Advances in Fiber-Optic Technology for Point-of-Care Diagnosis and In Vivo Biosensing. Adv. Mater. Technol. 2020, 5, 1900792. [Google Scholar] [CrossRef]
- Cai, S.; Pan, H.; González-Vila, Á.; Guo, T.; Gillan, D.C.; Wattiez, R.; Caucheteur, C. Selective detection of cadmium ions using plasmonic optical fiber gratings functionalized with bacteria. Opt. Express 2020, 28, 19740–19749. [Google Scholar] [CrossRef] [PubMed]
- Bachhuka, A.; Heng, S.; Vasilev, K.; Kostecki, R.; Abell, A.; Ebendorff-Heidepriem, H. Surface Functionalization of Exposed Core Glass Optical Fiber for Metal Ion Sensing. Sensors 2019, 19, 1829. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, X.; Hu, X.; Song, S.; Fan, C. Unmodified gold nanoparticles as a colorimetric probe for potassium DNA aptamers. Chem. Commun. 2006, 3780–3782. [Google Scholar] [CrossRef] [PubMed]
- Rehman, F.N.; Audeh, M.; Abrams, E.S.; Hammond, P.W.; Kenney, M.; Boles, T.C. Immobilization of acrylamide-modified oligonucleotides by co-polymerization. Nucleic Acids Res. 1999, 27, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Damase, T.R.; Ellington, A.D.; Allen, P.B. Purification of single-stranded DNA by co-polymerization with acrylamide and electrophoresis. Biotechniques 2017, 62, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Tierney, S.; Hjelme, D.R.; Stokke, B.T. Determination of swelling of responsive gels with nanometer resolution. Fiber-optic based platform for hydrogels as signal transducers. Anal. Chem. 2008, 80, 5086–5093. [Google Scholar] [CrossRef]
- Nzihou, A.; Sharrock, P. Role of Phosphate in the Remediation and Reuse of Heavy Metal Polluted Wastes and Sites. Waste Biomass Valorization 2010, 1, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Blais, J.F.; Djedidi, Z.; Cheikh, R.B.; Tyagi, R.D.; Mercier, G. Metals Precipitation from Effluents: Review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 135–149. [Google Scholar] [CrossRef]
- Lewenstam, A. Routines and Challenges in Clinical Application of Electrochemical Ion-Sensors. Electroanalysis 2014, 26, 1171–1181. [Google Scholar] [CrossRef]
- Li, H.; Sun, S.R.; Yap, J.Q.; Chen, J.H.; Qian, Q. 0.9% saline is neither normal nor physiological. J. Zhejiang Univ. Sci. B 2016, 17, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciobanu, M.; Wilburn, J.P.; Lowy, D.A. Miniaturized Reference Electrodes. II. Use in Corrosive, Biological, and Organic Media. Electroanalysis 2004, 16, 1351–1358. [Google Scholar] [CrossRef]
- Hjelme, D.R.; Berg, A.; Ellingsen, R.; Falch, B.; Bjørkøy, A.; Østling, D. Optical Sensing of Measurands. U.S. Patent 7,602,498, 13 October 2009. [Google Scholar]
- Tinland, B.; Pluen, A.; Sturm, J.; Weill, G. Persistence length of single-stranded DNA. Macromolecules 1997, 30, 5763–5765. [Google Scholar] [CrossRef]
- Goddard, N.L.; Bonnet, G.; Krichevsky, O.; Libchaber, A. Sequence dependent rigidity of single stranded DNA. Phys. Rev. Lett. 2000, 85, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, H.; Takagi, M.; Takenaka, S. A Novel Potassium Sensing in Aqueous Media with a Synthetic Oligonucleotide Derivative. Fluorescence Resonance Energy Transfer Associated with Guanine Quartet−Potassium Ion Complex Formation. J. Am. Chem. Soc. 2002, 124, 14286–14287. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U.P.; Oppermann, W. Properties of polyelectrolyte gels. In Physical Properties of Polymeric Gels; J. Wiley & Sons: Chichester, UK, 1996; pp. 19–38. [Google Scholar]
- Suárez, I.J.; Fernández-Nieves, A.; Márquez, M. Swelling kinetics of poly(N-isopropylacrylamide) minigels. J. Phys. Chem. B 2006, 110, 25729–25733. [Google Scholar] [CrossRef] [PubMed]

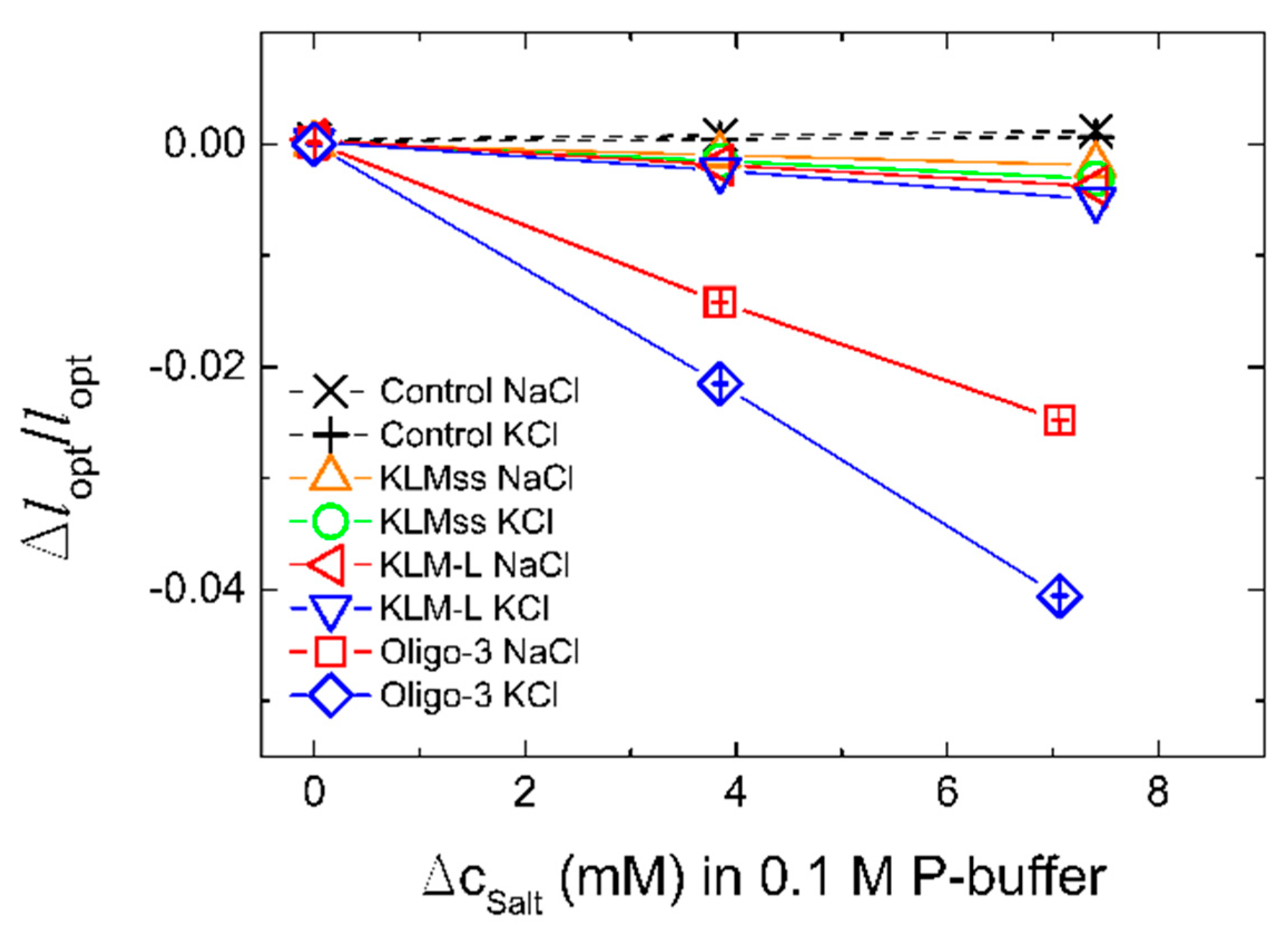
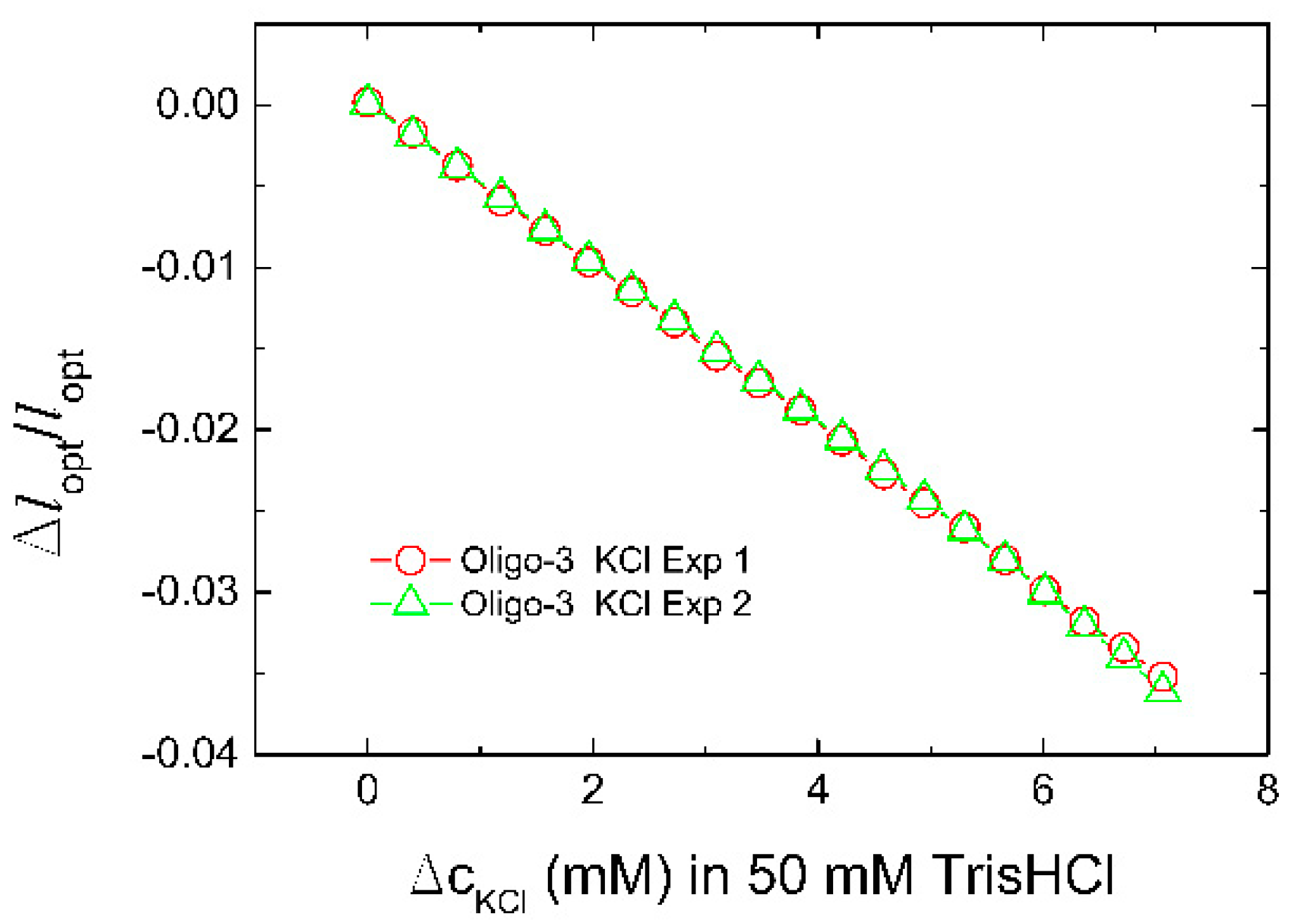
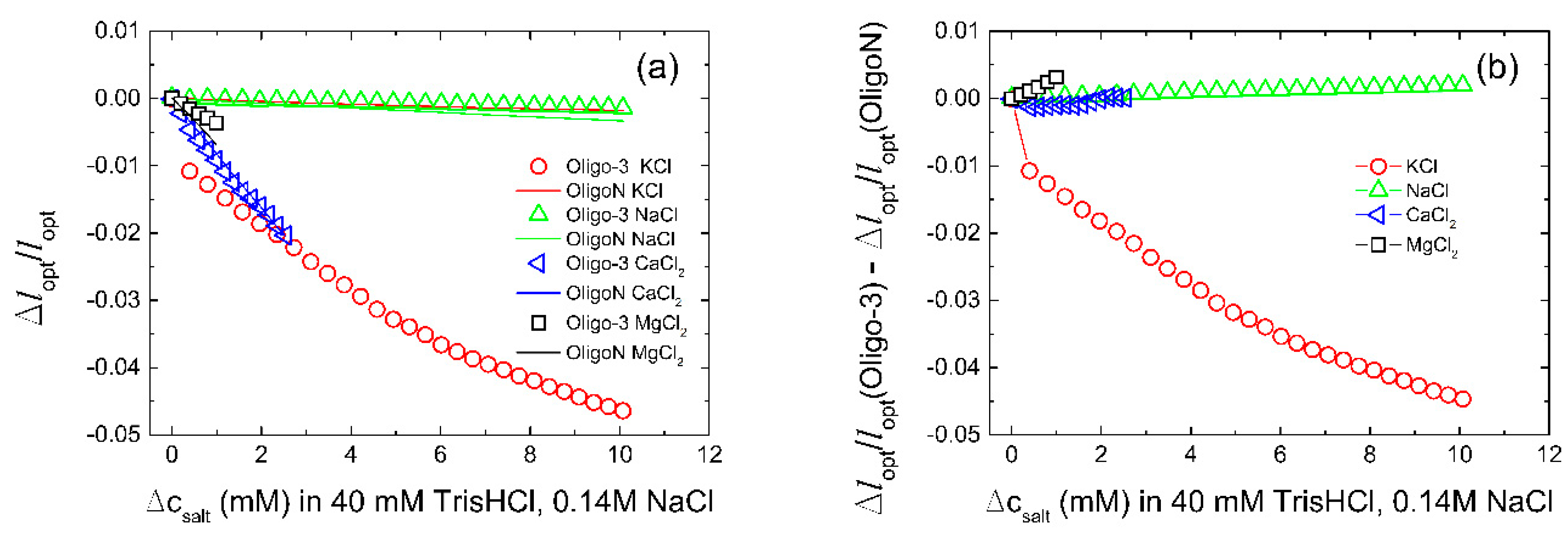
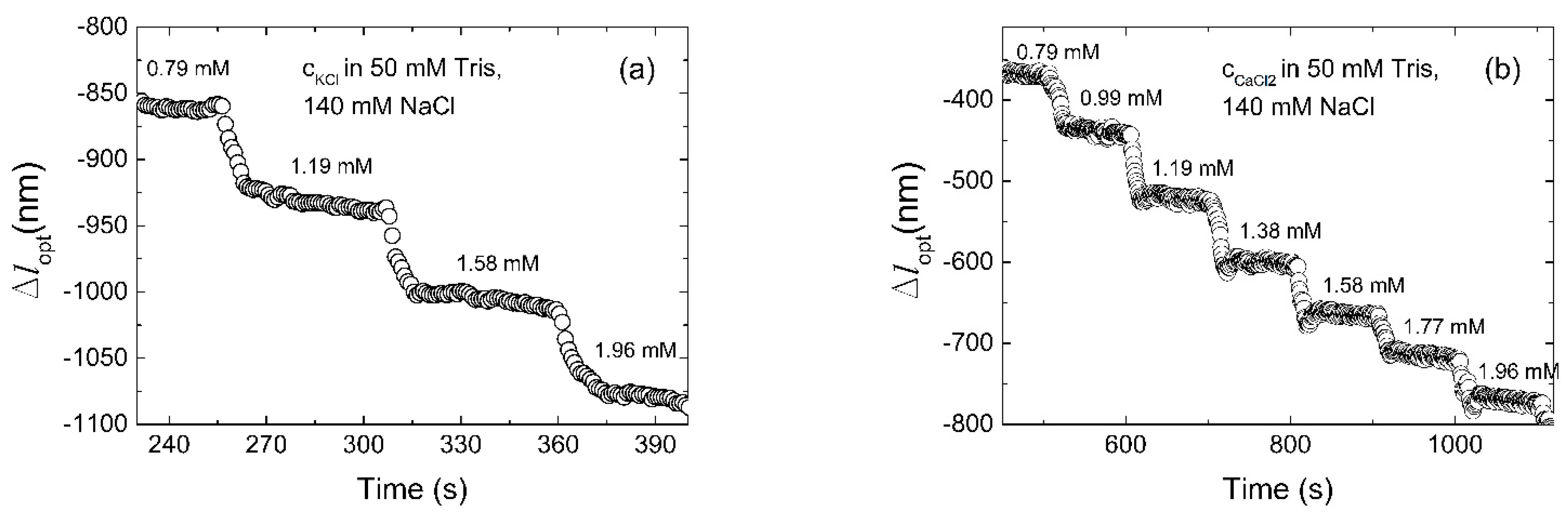

| Aptamer | Oligonucleotide Sequences |
|---|---|
| Oligo-3 | 5′- /Acryd/AAA ATG AGG GAG GGG -3′ |
| OligoN | 5′- /Acryd/AAA ATG GAC AAA CGA -3′ |
| KLM | 5′- /Acryd/AAA AGG GTT AGG GTT AGG GTT AGG GAA AAG CGT CCT CCG -3′ |
| L | 5′- /Acryd/AAA ACG GAG GAC GC -3′ |
| KLMss | 5′- /Acryd/AAA AGG GTT AGG GTT AGG GTT AGG G -3′ |
| Component | Normal Range | Average Level |
|---|---|---|
| NaCl | 135–145 mM | 140 mM |
| KCl | 3.60–5.20 mM | 4.40 mM |
| CaCl2 | 2.20–2.50 mM | 2.35 mM |
| MgCl2 | 0.70–1.00 mM | 0.85 mM |
| Albumin | 3.50–5.00 g/dL | 4.25 g/dL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žuržul, N.; Stokke, B.T. DNA Aptamer Functionalized Hydrogels for Interferometric Fiber-Optic Based Continuous Monitoring of Potassium Ions. Biosensors 2021, 11, 266. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11080266
Žuržul N, Stokke BT. DNA Aptamer Functionalized Hydrogels for Interferometric Fiber-Optic Based Continuous Monitoring of Potassium Ions. Biosensors. 2021; 11(8):266. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11080266
Chicago/Turabian StyleŽuržul, Nataša, and Bjørn Torger Stokke. 2021. "DNA Aptamer Functionalized Hydrogels for Interferometric Fiber-Optic Based Continuous Monitoring of Potassium Ions" Biosensors 11, no. 8: 266. https://0-doi-org.brum.beds.ac.uk/10.3390/bios11080266






