Acetone Gas Sensor Based on SWCNT/Polypyrrole/Phenyllactic Acid Nanocomposite with High Sensitivity and Humidity Stability
Abstract
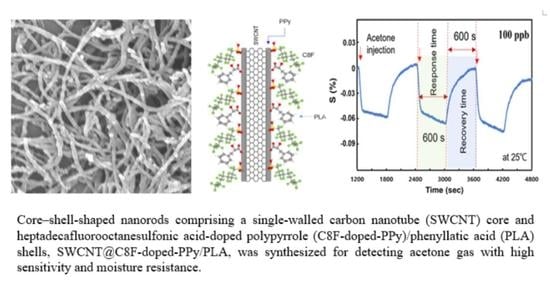
:1. Introduction
2. Materials and Methods
2.1. Synthesis of C8F-Doped-PPy/PLA@SWCNT
2.2. Structural Analysis and Measurement of Gas Sensors
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Luo, J.; Wang, L.; Shi, X.; Yin, J.; Crew, E.; Lu, S.; Lesperance, L.M.; Zhong, C.-J. Nanoparticle-structured thin film sensor arrays for breath sensing. Sens. Actuators B 2012, 161, 845–854. [Google Scholar] [CrossRef]
- Chuang, M.Y.; Lin, Y.T.; Tung, T.W.; Chang, L.Y.; Zan, H.W.; Meng, H.F.; Lu, C.J.; Tao, Y.T. Room-temperature-operated organic-based acetone gas sensor for breath analysis. Sens. Actuators B 2018, 260, 593–600. [Google Scholar] [CrossRef]
- Yu, Y.T.; Dutta, P. Examination of Au/SnO2 core-shell architecture nanoparticle for low temperature gas sensing applications. Sens. Actuators B 2011, 157, 444–449. [Google Scholar] [CrossRef]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef]
- Jang, J.S.; Choi, S.J.; Kim, S.J.; Hakim, M.; Kim, I.D. Rational Design of Highly Porous SnO2 Nanotubes Functionalized with Biomimetic Nanocatalysts for Direct Observation of Simulated Diabetes. Adv. Funct. Mater. 2016, 26, 4740–4748. [Google Scholar] [CrossRef]
- Parkes, J.L.; Slatin, S.L.; Pardo, S.; Ginsberg, B.H.A. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care 2000, 23, 1143–1148. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.C. Measuring breath acetone for monitoring fat loss: Review. Obes. Soc. 2015, 23, 2327–2334. [Google Scholar] [CrossRef]
- Turner, C.; Walton, C.C.; Hoashi, S.; Evans, M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J. Breath Res. 2009, 3, 046004. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C. Is breath acetone a biomarker of diabetes? A historical review on breath acetone measurements. J. Breath Res. 2013, 7, 037109. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Zhang, D.; Zhang, L.; Lu, G. Non-invasive blood glucose monitoring for diabetics by means of breath signal analysis. Sens. Actuators B 2012, 173, 106–113. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured Materials for Room-Temperature Gas Sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, L.; Sheng, K.; Zhou, X.; Dong, B.; Lu, G.; Song, H. A highly sensitive and moisture-resistant gas sensor for diabetes diagnosis with Pt@In2O3 nanowires and a molecular sieve for protection. NPG Asia Mater. 2018, 10, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Zheng, M.R. Preparation of high-aspect-ratio ZnO nanorod arrays for the detection of several organic solvents at room working temperature. Appl. Surf. Sci. 2013, 285, 241–248. [Google Scholar] [CrossRef]
- Xing, R.; Li, Q.; Xia, L.; Song, J.; Xu, L.; Zhang, J.; Xie, Y.; Song, H. Au-modified three-dimensional In2O3 inverse opals: Synthesis and improved performance for acetone sensing toward diagnosis of diabetes. Nanoscale 2015, 7, 13051–13060. [Google Scholar] [CrossRef] [Green Version]
- Khun, K.K.; Mahajan, A.; Bedi, R.K. SnO2 thick films for room temperature gas sensing applications. J. Appl. Phys. 2009, 106, 124509. [Google Scholar] [CrossRef]
- Shi, J.; Hu, G.; Sun, Y.; Geng, M.; Wu, J.; Liu, Y.; Ge, M.; Tao, J.; Cao, M.; Dai, N. WO3 nanocrystals: Synthesis and application in highly sensitive detection of acetone. Sens. Actuators B 2011, 156, 820–824. [Google Scholar] [CrossRef]
- Cherenack, K.; Zysset, C.; Kinkeldei, T.; Münzenrieder, N.; Tröster, G. Woven Electronic Fibers with Sensing and Display Functions for Smart Textiles. Adv. Mater. 2010, 22, 5178–5182. [Google Scholar] [CrossRef]
- Zhang, R.; Deng, H.; Valenca, R.; Jin, J.; Fu, Q.; Bilotti, E.; Peijs, T. Strain sensing behaviour of elastomeric composite films containing carbon nanotubes under cyclic loading. Compos. Sci. Technol. 2013, 74, 1–5. [Google Scholar] [CrossRef]
- Hua, C.; Shang, Y.; Wang, Y.; Xu, J.; Zhang, Y.; Li, X.; Cao, A. A flexible gas sensor based on single-walled carbon nanotube-Fe2O3 composite film. Appl. Surf. Sci. 2017, 405, 405–411. [Google Scholar] [CrossRef]
- Du, W.X.; Lee, H.J.; Byeon, J.H.; Kim, J.S.; Cho, K.S.; Kang, S.; Takada, M.; Kim, J.Y. Highly sensitive single-walled carbon nanotube/polypyrrole/phenylalanine core–shell nanorods for ammonia gas sensing. J. Mater. Chem. C 2020, 8, 15609–15615. [Google Scholar]
- Kumar, L.; Rawal, I.; Kaur, A. Flexible room temperature ammonia sensor based on polyaniline. Sens. Actuators B 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Ihm, D.W.; Woo, H.Y.; Hwang, C.R.; Lee, Y.K.; Kim, J.Y. Fabrication of polypyrrole–phenylalanine nano-films with NH3 gas sensitivity. Sens. Actuators B 2011, 153, 421–426. [Google Scholar] [CrossRef]
- Liu, H.; Kameoka, J.; Czaplewski, D.A.; Craighead, H.G. Polymeric Nanowire Chemical Sensor. Nano Lett. 2004, 4, 671–675. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Wang, K.; Wei, J.; Wu, D.; Cao, A.; Li, Z.; Cheng, Y.; Zheng, Q. Stretchable and highly sensitive graphene-on-polymer strain sensors. Sci. Rep. 2012, 16, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Hernandez, L.; Potje-Kamloth, K. Optical and electrical characterization of a conducting polypyrrole composite prepared by insitu electropolymerization. Phys. Chem. Chem. Phys. 1999, 1, 1735–1742. [Google Scholar] [CrossRef]
- Demoustier-Champagne, S.; Stavaux, P.Y. Effect of electrolyte concentration and nature on the morphology and the electrical properties of electropolymerized polypyrrole nanotubules. Chem. Mater. 1999, 11, 829–834. [Google Scholar] [CrossRef]
- Liu, Y.C. Characteristics of vibration modes of polypyrrole on surface-enhanced Raman scattering spectra. J. Electroanal. Chem. 2004, 571, 255–264. [Google Scholar] [CrossRef]
- Schmidt, J.; Dybal, J.; Rodriguez-Cabello, J.C.; Reboto, V. Role of water in structural changes of poly(AVGVP) and poly(GVGVP) studied by FTIR and Raman spectroscopy and ab iinitio calculations. Biomacromolecules 2005, 6, 697–706. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Jung, Y.C.; So, H.H.; Cho, J.W. Polypyrrole coated carbon nanotubes: Synthesis, characterization, and enhanced electrical properties. Synth. Met. 2007, 157, 374–379. [Google Scholar] [CrossRef]
- Kuan, H.C.; Ma, C.M.; Chang, W.P.; Yuen, S.M.; Wu, H.H.; Lee, T.M. Synthesis, thermal, mechanical and rheological properties of multiwall carbon nanotube/waterborne polyurethane nanocomposite. Compos. Sci. Technol. 2005, 65, 1703–1710. [Google Scholar] [CrossRef]
- Van Hieu, N.; Dung, N.Q.; Tam, P.D.; Trung, T.; Chien, N.D. Thin film polypyrrole/SWCNTs nanocomposites-based NH3 sensor operated at room temperature. Sens. Actuators B 2009, 140, 500–507. [Google Scholar] [CrossRef]
- Jang, W.K.; Kim, J.Y.H.; Lee, Y.S. Improvement in ammonia gas sensing behavior by polypyrrole/multi-walled carbon nanotubes composites. Carbon Lett. 2012, 13, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Kawashima, H.; Mayama, H.; Nakamura, Y.; Fujii, S. Hydrophobic polypyrroles synthesized by aqueous chemical oxidative polymerization and their use as light-responsive liquid marble stabilizers. Polym. Chem. 2017, 8, 2609–2618. [Google Scholar] [CrossRef]
- Jaeglé, L.; Jacob, D.J.; Brune, W.H.; Faloona, I.; Tan, D.; Heikes, B.G.; Kondo, Y.; Sachse, G.W.; Anderson, B.; Shetter, R.E. Photochemistry of HOx in the upper troposphere at northern midlatitudes. J. Geophys. Res. Atmos. 2000, 105, 3877–3892. [Google Scholar] [CrossRef]
- Cyran, J.D.; Backus, E.H.G.; van Zadel, M.-J.; Bonn, M. Comparative adsorption of acetone on water and ice surfaces. Angew. Chem. Int. Ed. 2019, 58, 3620–3624. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byeon, J.-H.; Kim, J.-S.; Kang, H.-K.; Kang, S.; Kim, J.-Y. Acetone Gas Sensor Based on SWCNT/Polypyrrole/Phenyllactic Acid Nanocomposite with High Sensitivity and Humidity Stability. Biosensors 2022, 12, 354. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12050354
Byeon J-H, Kim J-S, Kang H-K, Kang S, Kim J-Y. Acetone Gas Sensor Based on SWCNT/Polypyrrole/Phenyllactic Acid Nanocomposite with High Sensitivity and Humidity Stability. Biosensors. 2022; 12(5):354. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12050354
Chicago/Turabian StyleByeon, Jun-Ho, Ji-Sun Kim, Hyo-Kyung Kang, Sungmin Kang, and Jin-Yeol Kim. 2022. "Acetone Gas Sensor Based on SWCNT/Polypyrrole/Phenyllactic Acid Nanocomposite with High Sensitivity and Humidity Stability" Biosensors 12, no. 5: 354. https://0-doi-org.brum.beds.ac.uk/10.3390/bios12050354