Automatic Spot Identification Method for High Throughput Surface Plasmon Resonance Imaging Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Video Accessing and Parallel Processing
2.2.2. Morphological Filtering
2.2.3. Image Sharpening
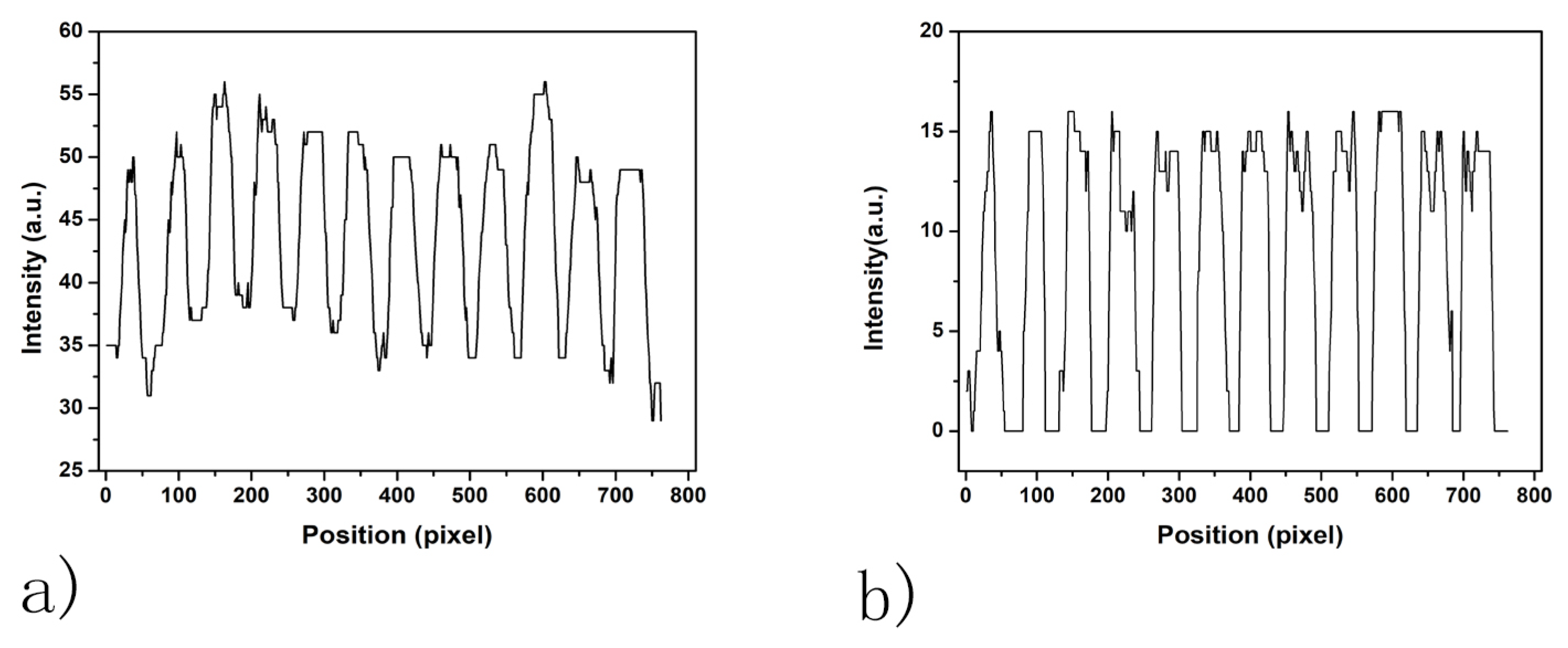
2.2.4. Adaptive Threshold Binarization
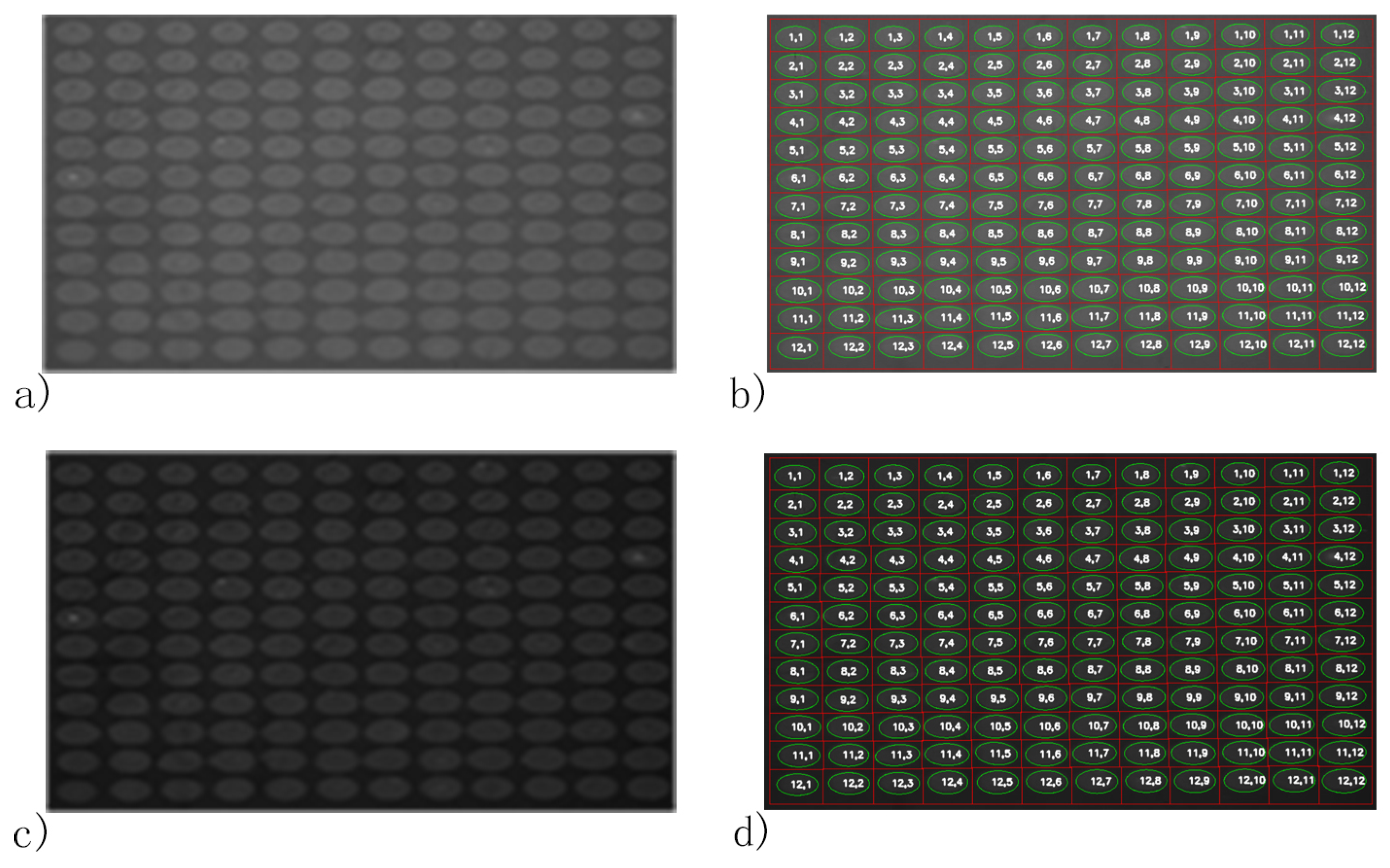
2.2.5. Contour Detection, Ellipse Fitting and Spot Addressing
2.2.6. Spot Merging
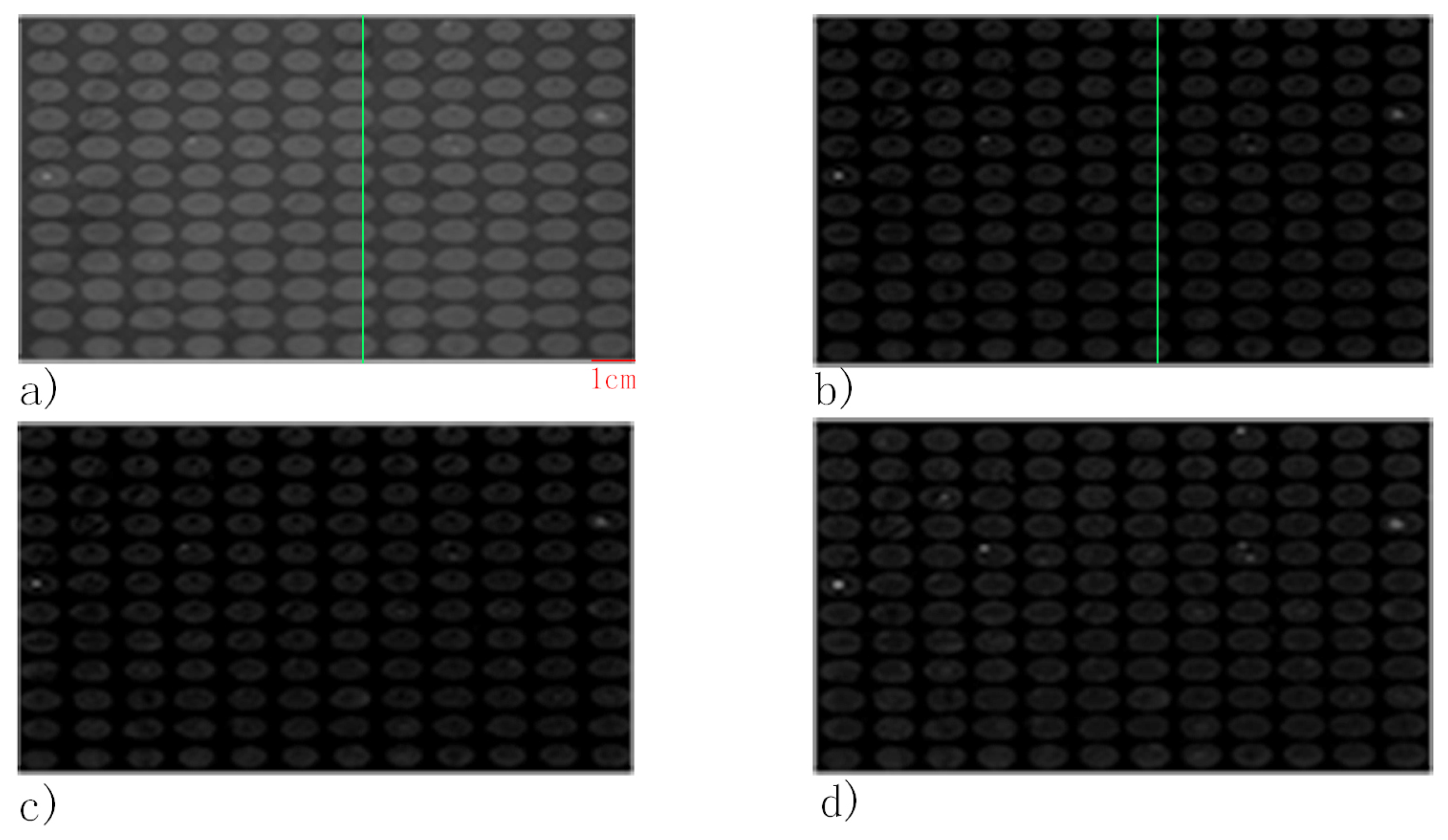
3. Results and Discussion
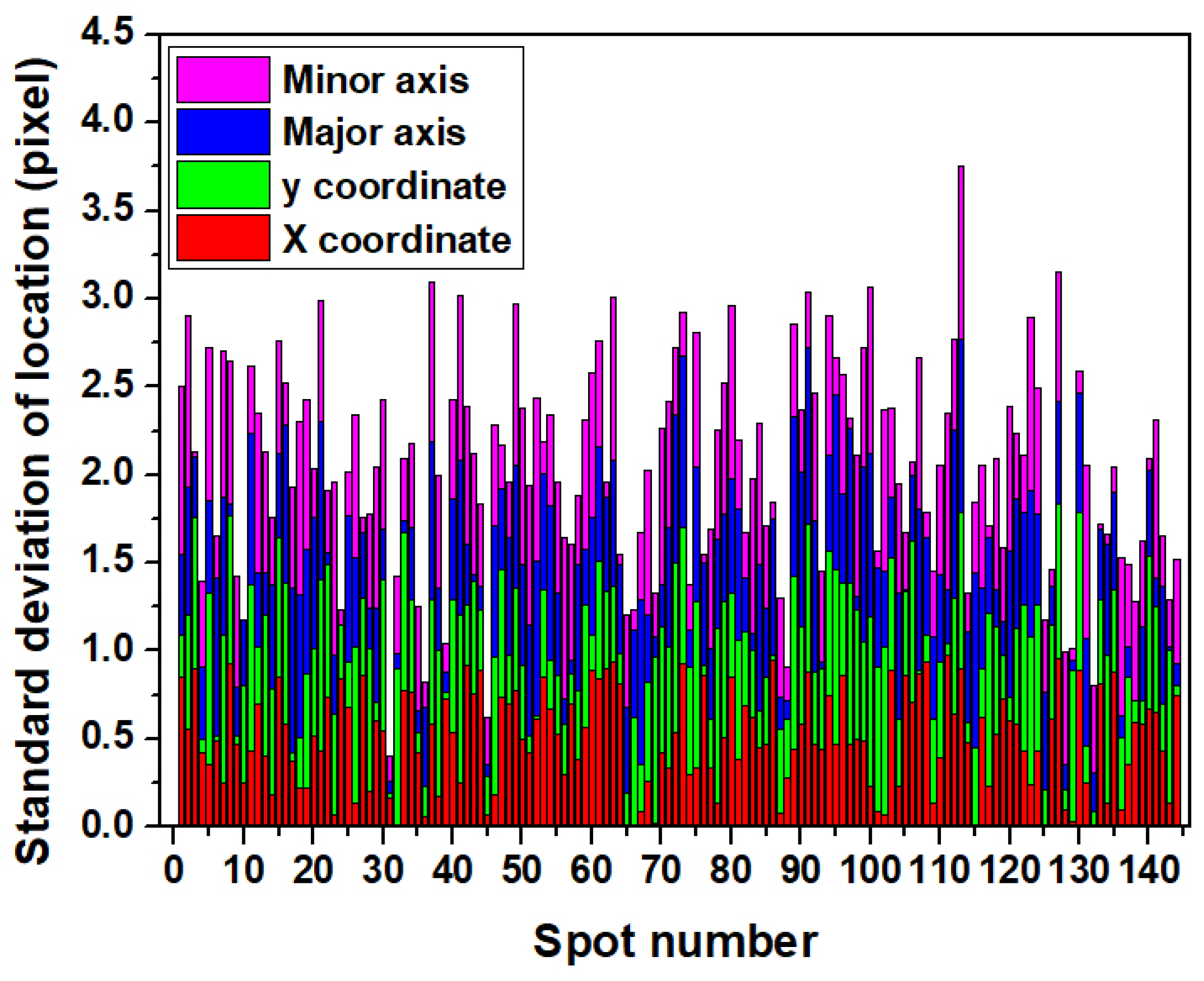
3.1. FKBP12 Microarray
3.2. Rabbit IgG Microarray
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yoon, K.J.; Song, G.; Qian, X.; Pan, J.; Xu, D.; Rho, H.S.; Kim, N.S.; Habela, C.; Zheng, L.; Jacob, F.; et al. Zika-virus-encoded NS2A disrupts mammalian cortical neurogenesis by degrading adherens junction proteins. Cell Stem Cell 2017, 21, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, F.S.H.; Sutandy, F.R.; Syu, G.D.; Chen, Y.W.; Lin, J.M.; Chen, C.S. Systematic protein interactome analysis of glycosaminoglycans revealed YcbS as a novel bacterial virulence factor. Sci. Rep. 2016, 6, 28425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, I.; Simizu, S.; Okumura, H.; Takagi, S.; Osada, H. A small-molecule inhibitor shows that pirin regulates migration of melanoma cells. Nat. Chem. Biol. 2010, 6, 667. [Google Scholar] [CrossRef] [PubMed]
- Bradner, J.E.; McPherson, O.M.; Mazitschek, R.; Barnes-Seeman, D.; Shen, J.P.; Dhaliwal, J.; Stevenson, K.E.; Duffner, J.L.; Park, S.B.; Neuberg, D.S.; et al. A robust small-molecule microarray platform for screening cell lysates. Chem. Biol. 2006, 13, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Rho, H.S.; Pan, J.; Ramos, P.; Yoon, K.J.; Medina, F.A.; Lee, E.M.; Eichinger, D.J.; Ming, G.L.; Muñoz-Jordan, J.L.; et al. Multiplexed biomarker panels discriminate Zika and Dengue virus infection in humans. Mol. Cell. Proteom. 2017, RA117. [Google Scholar] [CrossRef]
- Venkataraman, A.; Yang, K.; Irizarry, J.; Mackiewicz, M.; Mita, P.; Kuang, Z.; Xue, L.; Ghosh, D.; Liu, S.; Ramos, P.; et al. A toolbox of immunoprecipitation-grade monoclonal antibodies to human transcription factors. Nat. Methods 2018, 15, 330. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Syu, G.D.; Chung, K.H.; Ho, Y.H.; Chung, F.H.; Chen, P.H.; Lin, J.M.; Chen, Y.W.; Tsai, S.Y.; Chen, C.S. Antibody profiling of bipolar disorder using Escherichia coli proteome microarrays. Mol. Cell. Proteom. 2014, M114. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.T.; Kim, G. SPR microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials. Biomaterials 2008, 28, 2380. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.P.; Grimsrud, T.E.; Liles, M.R.; Goodman, R.M.; Corn, R.M. Surface plasmon resonance imaging measurements of DNA and RNA hybridization adsorption onto DNA microarrays. Anal. Chem. 2001, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Cui, Y.; Cheng, Z.Q.; Song, L.S.; Wang, Z.Y.; Han, B.H.; Zhu, J.S. Poly (acrylic acid) brushes pattern as a 3D functional biosensor surface for microchips. Appl. Surf. Sci. 2013, 266, 313–318. [Google Scholar] [CrossRef]
- Piliarik, M.; Bocková, M.; Homola, J. Surface plasmon resonance biosensor for parallelized detection of protein biomarkers in diluted blood plasma. Biosens. Bioelectron. 2010, 26, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Lausted, C.; Hu, Z.; Hood, L. Quantitative serum proteomics from surface plasmon resonance imaging. Mol. Cell. Proteom. 2008, 7, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Castiello, F.R.; Tabrizian, M. Multiplex Surface Plasmon Resonance Imaging-Based Biosensor for Human Pancreatic Islets Hormones Quantification. Anal. Chem. 2018, 90, 3132–3139. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, N.; Kyo, M.; Inamori, K.; Ando, A.; Asami, A.; Nakao, A.; Osada, H. SPR imaging of photo-cross-linked small-molecule arrays on gold. Anal. Chem. 2006, 78, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yang, M.; Zhou, W.; Zhang, B.; Cheng, Z.; Huang, J.; Zhang, M.; Wang, Z.; Wang, R.; Chen, Z.; et al. Kinetic and high-throughput profiling of epigenetic interactions by 3D-carbene chip-based surface plasmon resonance imaging technology. Proc. Natl. Acad. Sci. USA 2017, 114, E7245–E7254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, C.; Cheng, Z.; Zhang, D.; Li, S.; Song, L.; Zhou, W.; Yang, M.; Wang, Z.; Zheng, Z.; et al. Spri determination of inter-peptide interaction by using 3D supramolecular co-assembly polyrotaxane film. Biosens. Bioelectron. 2015, 66, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, W.; Bu, X.; Wei, Z.; Geng, L.; Wu, Y.; Dong, C.; Li, L.; Zhang, D.; Yang, S.; et al. Microarray based screening of peptide nano probes for HER2 positive tumor. Anal. Chem. 2015, 2015 87, 8367–8372. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Z.; Cheng, P.; He, Z.; Cheng, Z.; Peng, J.; Wang, H.; Wang, C.; Yang, Y.; Hu, Z. Antibody-Mimetic Peptoid Nanosheet for Label-Free Serum-Based Diagnosis of Alzheimer’s Disease. Adv. Mater. 2017, 29, 1700057. [Google Scholar] [CrossRef] [PubMed]
- Abali, F.; Stevens, M.; Tibbe, A.G.J.; Terstappen, L.W.M.M.; van der Velde, P.N.; Schasfoort, R.B.M. Isolation of single cells for protein therapeutics using microwell selection and Surface Plasmon Resonance imaging. Anal. Biochem. 2017, 531, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar]
- Oh, Y.; Choi, J.R.; Lee, W.; Kim, D. Surface Plasmon-Enhanced Super-Localization Microscopy. In Handbook of Photonics for Biomedical Engineering; Springer: Amsterdam, The Netherlands, 2017; pp. 365–381. [Google Scholar]
- Díez, P.; Dasilva, N.; González-González, M.; Matarraz, S.; Casado-Vela, J.; Orfao, A.; Fuentes, M. Data analysis strategies for protein microarrays. Microarrays 2012, 2, 64–83. [Google Scholar]
- Boellner, S.; Becker, K.F. Reverse phase protein arrays—Quantitative Assessment of Multiple Biomarkers in Biopsies for Clinical Use. Microarrays 2015, 4, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yu, J.; Tan, J. Direct inverse randomized Hough transform for incomplete ellipse detection in noisy images. JPRR 2014, 1, 13–24. [Google Scholar] [CrossRef]
- Wu, E.; Su, Y.A.; Billings, E.; Brooks, B.R.; Wu, X. Automatic spot identification for high throughput microarray analysis. JBBS 2011, 005. [Google Scholar]
- Wang, Z.; Cheng, Z.; Singh, V.; Zheng, Z.; Wang, Y.; Li, S.; Song, L.; Zhu, J. Stable and sensitive silver surface plasmon resonance imaging sensor using trilayered metallic structures. Anal. Chem. 2014, 86, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, Z.; Gillespie, D.E.; Lausted, C.; Zheng, Z.; Yang, M.; Zhu, J. Plain silver surface plasmon resonance for microarray application. Anal. Chem. 2015, 87, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Howse, J. OpenCV Computer Vision With Python; Packt Publishing Ltd.: Birmingham, UK, 2010; p. 667. [Google Scholar]
- Culjak, I.; Abram, D.; Pribanic, T.; Dzapo, H.; Cifrek, M. A brief introduction to OpenCV. In Proceedings of the 35th International Convention, Opatija, Croatia, 21–25 May 2012. [Google Scholar]
- Sawant, H.K.; Deore, M. A comprehensive review of image enhancement techniques. IJCTEE 2010, 1, 39–44. [Google Scholar]
- Bovik, A.C. Handbook of Image and Video Processing; Elsevier Academic Press: Amsterdam, The Netherland, 2005; pp. 135–156. [Google Scholar]
- Hailan, G.; Wenzhe, L. A Modified Homomorphic Filter for Image Enhancement. In Proceedings of the 2nd International Conference on Computer Application and System Modeling, Xiamen, China, 27–29 July 2012; pp. 176–180. [Google Scholar]
- Archana, J.N. A Review on the Image Sharpening Algorithms Using Unsharp Masking. Int. J. Eng. Sci. Comp. 2016, 6, 8729–8733. [Google Scholar]
- Prasad, S.; Ganesan, N. An Image Sharpening Method by Suppressing the Noise. Int. J. Comput. Appl. Technol. 2012, 51, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Bataineh, B.; Abdullah, S.N.H.S.; Omar, K.T. An adaptive local binarization method for document images based on a novel thresholding method and dynamic windows. Pattern Recognit. Lett. 2011, 32, 1805–1813. [Google Scholar] [CrossRef]
- Singh, T.R.; Roy, S.; Singh, O.I.; Sinam, T.; Singh, K. A new local adaptive thresholding technique in binarization. ArXiv Preprint, 2012; arXiv:1201.5227. [Google Scholar]
- Sadiq, B.O.; Sani, S.M.; Garba, S. Edge Detection: A Collection of Pixel based Approach for Colored Images. ArXiv Preprint, 2015; arXiv:1503.05689. [Google Scholar]
- Katiyar, S.K.; Arun, P.V. Comparative analysis of common edge detection a techniques in context of object extraction. IEEE TGRS 2014, 50, 68–79. [Google Scholar]
- Papari, G.; Petkov, N. Edge and line oriented contour detection: State of the art. Image Vis. Comput. 2011, 29, 79–103. [Google Scholar] [CrossRef]
- Mignotte, M. A biologically inspired framework for contour detection. Pattern Anal. Appl. 2017, 20, 365–381. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophoton. Int. 2004, 11, 36–42. [Google Scholar]





| X Coordinate (Pixel) | Y Coordinate (Pixel) | Major Axis (Pixel) | Minor Axis (Pixel) | |
|---|---|---|---|---|
| FK506 | −0.195 | −0.193 | −0.164 | 0.243 |
| Phosphorous Acid | −0.241 | 0.475 | 0.304 | −0.710 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Huang, X.; Cheng, Z. Automatic Spot Identification Method for High Throughput Surface Plasmon Resonance Imaging Analysis. Biosensors 2018, 8, 85. https://0-doi-org.brum.beds.ac.uk/10.3390/bios8030085
Wang Z, Huang X, Cheng Z. Automatic Spot Identification Method for High Throughput Surface Plasmon Resonance Imaging Analysis. Biosensors. 2018; 8(3):85. https://0-doi-org.brum.beds.ac.uk/10.3390/bios8030085
Chicago/Turabian StyleWang, Zhiyou, Xiaoqing Huang, and Zhiqiang Cheng. 2018. "Automatic Spot Identification Method for High Throughput Surface Plasmon Resonance Imaging Analysis" Biosensors 8, no. 3: 85. https://0-doi-org.brum.beds.ac.uk/10.3390/bios8030085




