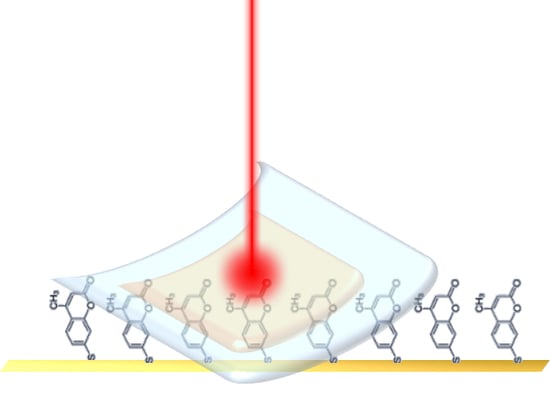
Flexible and Transparent Substrates Based on Gold Nanoparticles and TiO2 for in Situ Bioanalysis by Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Gold Nanoparticles Preparation
2.3. Preparation of Pesticides Standard Suspensions and Solutions
2.4. Flexible SERS Substrate Preparation
2.5. SEM Imaging
2.6. SERS Mapping Measurements
2.7. SERS Characterization Tests
2.8. Enhancement Factor Calculation
3. Results and Discussion
3.1. Flexible SERS Substrate Optimization
3.2. SERS Substrate Characterization
3.3. Homogeneity of Response in Transmission Configuration
3.4. Simulation of Application of the SERS Substrate for Pesticides Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hudson, S.D.; Chumanov, G. Bioanalytical applications of SERS (surface-enhanced Raman spectroscopy). Anal. Bioanal. Chem. 2009, 394, 679–686. [Google Scholar] [CrossRef] [PubMed]
- McNay, G.; Eustace, D.; Smith, W.E.; Faulds, K.; Graham, D. Surface-Enhanced Raman Scattering (SERS) and Surface-Enhanced Resonance Raman Scattering (SERRS): A Review of Applications. Appl. Spectrosc. 2011, 65, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, L. Surface-Enhanced Raman Spectroscopy for the Chemical Analysis of Food. Compr. Rev. Food Sci. Food Saf. 2014, 13, 317–328. [Google Scholar] [CrossRef]
- Mandrile, L.; Giovannozzi, A.M.; Durbiano, F.; Martra, G.; Rossi, A.M. Rapid and sensitive detection of pyrimethanil residues on pome fruits by Surface Enhanced Raman Scattering. Food Chem. 2018, 244, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M.-Y. Shell Thickness-Dependent Raman Enhancement for Rapid Identification and Detection of Pesticide Residues at Fruit Peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef]
- Luo, H.; Huang, Y.; Lai, K.; Rasco, B.A.; Fan, Y. Surface-enhanced Raman spectroscopy coupled with gold nanoparticles for rapid detection of phosmet and thiabendazole residues in apples. Food Control 2016, 68, 229–235. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Pergolese, B.; Bigotto, A.; Giusti, A. Stable and efficient silver substrates for SERS spectroscopy. J. Colloid Interface Sci. 2007, 314, 540–544. [Google Scholar] [CrossRef]
- Abu Hatab, N.A.; Oran, J.M.; Sepaniak, M.J. Surface-enhanced Raman spectroscopy substrates created via electron beam lithography and nanotransfer printing. ACS Nano 2008, 2, 377–385. [Google Scholar] [CrossRef]
- Sharma, B.; Cardinal, M.F.; Kleinman, S.L.; Greeneltch, N.G.; Frontiera, R.R.; Blaber, M.G.; Schatz, G.C.; Van Duyne, R.P. High-performance SERS substrates: Advances and challenges. MRS Bull. 2013, 38, 615–624. [Google Scholar] [CrossRef] [Green Version]
- Mosier-Boss, P.A. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [Green Version]
- Polavarapu, L.; Liz-Marzan, L.M. Towards low-cost flexible substrates for nanoplasmonic sensing. Phys. Chem. Chem. Phys. 2013, 15, 5288–5300. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Zhu, K.; Gu, C.; Wen, Y.; Cui, G.; Qu, J. Transparent, flexible surface enhanced Raman scattering substrates based on Ag-coated structured PET (polyethylene terephthalate) for in-situ detection. Appl. Surf. Sci. 2016, 379, 66–72. [Google Scholar] [CrossRef]
- Zhong, L.-B.; Yin, J.; Zheng, Y.-M.; Liu, Q.; Cheng, X.-X.; Luo, F.-H. Self-Assembly of Au Nanoparticles on PMMA Template as Flexible, Transparent, and Highly Active SERS Substrates. Anal. Chem. 2014, 86, 6262–6267. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.D.; Kalb, W.B.; Chan, L.; Killian, M.G.; Bloch, J.-F.; Mazzeo, B.A.; Whitesides, G.M. Paper-Based, Capacitive Touch Pads. Adv. Mater. 2012, 24, 2850–2856. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Ahn, B.Y.; Adams, J.J.; Duoss, E.B.; Bernhard, J.T.; Lewis, J.A. Pen-on-Paper Flexible Electronics. Adv. Mater. 2011, 23, 3426–3430. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Goel, P.; Singh, J.P. Flexible and robust SERS active substrates for conformal rapid detection of pesticide residues from fruits. Sens. Actuators B-Chem. 2017, 241, 577–583. [Google Scholar] [CrossRef]
- Yea, K.; Lee, S.; Kyong, J.B.; Choo, J.; Lee, E.K.; Joo, S.W.; Lee, S. Ultra-sensitive trace analysis of cyanide water pollutant in a PDMS microfluidic channel using surface-enhanced Raman spectroscopy. Analyst 2005, 130, 1009–1011. [Google Scholar] [CrossRef]
- Singh, J.P.; Chu, H.; Abell, J.; Tripp, R.A.; Zhao, Y. Flexible and mechanical strain resistant large area SERS active substrates. Nanoscale 2012, 4, 3410–3414. [Google Scholar] [CrossRef]
- Suresh, V.; Yap, F.L. Flexible, transparent and robust SERS tapes through a two-step block copolymer self-assembly process. RSC Adv. 2015, 5, 61671–61677. [Google Scholar] [CrossRef]
- Lin, X.; Hasi, W.-L.-J.; Han, S.-Q.-G.-W.; Lou, X.-T.; Lin, D.-Y.; Lu, Z.-W. Fabrication of transparent SERS platform via interface self-assembly of gold nanorods and gel trapping technique for on-site real time detection. Phys. Chem. Chem. Phys. 2015, 17, 31324–31331. [Google Scholar] [CrossRef]
- Wen, X.; Li, G.; Zhang, J.; Zhang, Q.; Peng, B.; Wong, L.M.; Wang, S.; Xiong, Q. Transparent free-standing metamaterials and their applications in surface-enhanced Raman scattering. Nanoscale 2014, 6, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Aksu, S.; Huang, M.; Artar, A.; Yanik, A.A.; Selvarasah, S.; Dokmeci, M.R.; Altug, H. Flexible Plasmonics on Unconventional and Nonplanar Substrates. Adv. Mater. 2011, 23, 4422–4430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, X.; Xu, Y.; Wang, S.; Sun, T.; Liu, S.; Wu, X.; Xu, Z. Polymer nanopillar array with Au nanoparticle inlays as a flexible and transparent SERS substrate. RSC Adv. 2016, 6, 35527–35531. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, L.; Zhang, L.; Jing, C.; Shi, X.; Yang, Z.; Long, Y.; Fang, J. Large-area fabrication of highly reproducible surface enhanced Raman substrate via a facile double sided tape-assisted transfer approach using hollow Au-Ag alloy nanourchins. Nanoscale 2014, 6, 2567–2572. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.; Shen, B. Large-scale gold nanoparticle superlattice and its SERS properties for the quantitative detection of toxic carbaryl. Nanoscale 2013, 5, 5274–5278. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, M.; Li, H.; Li, M.; Zhang, C.; Shen, W.; Li, Y.; Guo, D.; Song, Y. Highly reproducible SERS arrays directly written by inkjet printing. Nanoscale 2015, 7, 421–425. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, T.; Sheng, K.; Zeng, L.; Ye, C.; Yu, T.; Zheng, X. Effect of pyrimethanil on Cryptococcus laurentii, Rhodosporidium paludigenum, and Rhodotorula glutinis biocontrol of Penicillium expansum infection in pear fruit. Int. J. Food Microbiol. 2013, 164, 155–160. [Google Scholar] [CrossRef]
- Gea, M.; Bonetta, S.; Iannarelli, L.; Giovannozzi, A.M.; Maurino, V.; Bonetta, S.; Hodoroaba, V.-D.; Armato, C.; Rossi, A.M.; Schiliro, T. Shape-engineered titanium dioxide nanoparticles (TiO2-NPs): Cytotoxicity and genotoxicity in bronchial epithelial cells. Food Chem. Toxicol. 2019, 127, 89–100. [Google Scholar] [CrossRef]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, E.; Shao, Y.; Xiao, Z.; Dong, Q.; Huang, J. Scalable fabrication of efficient organolead trihalide perovskite solar cells with doctor-bladed active layers. Energy Environ. Sci. 2015, 8, 1544–1550. [Google Scholar] [CrossRef]
- Ding, I.-K.; Melas-Kyriazi, J.; Cevey-Ha, N.-L.; Chittibabu, K.G.; Zakeeruddin, S.M.; Graetzel, M.; McGehee, M.D. Deposition of hole-transport materials in solid-state dye-sensitized solar cells by doctor-blading. Org. Electron. 2010, 11, 1217–1222. [Google Scholar] [CrossRef]
- Schmidt, M.S.; Hubner, J.; Boisen, A. Large Area Fabrication of Leaning Silicon Nanopillars for Surface Enhanced Raman Spectroscopy. Adv. Mater. 2012, 24, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman-spectrum of anatase, TIO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Cara, E.; Mandrile, L.; Lupi, F.F.; Giovannozzi, A.M.; Dialameh, M.; Portesi, C.; Sparnacci, K.; De Leo, N.; Rossi, A.M.; Boarino, L. Influence of the long-range ordering of gold-coated Si nanowires on SERS. Sci. Rep. 2018, 8, 11305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kara, S.A.; Keffous, A.; Giovannozzi, A.M.; Rossi, A.M.; Cara, E.; D’Ortenzi, L.; Sparnacci, K.; Boarino, L.; Gabouze, N.; Soukane, S. Fabrication of flexible silicon nanowires by self-assembled metal assisted chemical etching for surface enhanced Raman spectroscopy. RSC Adv. 2016, 6, 93649–93659. [Google Scholar] [CrossRef]
- Frey, S.; Stadler, V.; Heister, K.; Eck, W.; Zharnikov, M.; Grunze, M.; Zeysing, B.; Terfort, A. Structure of thioaromatic self-assembled monolayers on gold and silver. Langmuir 2001, 17, 2408–2415. [Google Scholar] [CrossRef]
- Caridad, J.M.; Winters, S.; McCloskey, D.; Duesberg, G.S.; Donegan, J.F.; Krstic, V. Hot-Volumes as Uniform and Reproducible SERS-Detection Enhancers in Weakly-Coupled Metallic Nanohelices. Sci. Rep. 2017, 7, 45548. [Google Scholar] [CrossRef] [Green Version]
- Ling, D.; Wu, Z.; Li, S.; Zhao, W.; Ma, C.; Wang, J.; Jiang, Z.; Zhong, Z.; Zheng, Y.; Yang, X. Large-Area Au-Nanoparticle-Functionalized Si Nanorod Arrays for Spatially Uniform Surface-Enhanced Raman Spectroscopy. ACS Nano 2017, 11, 1478–1487. [Google Scholar]
- Chen, H.-Y.; Lin, M.-H.; Wang, C.-Y.; Chang, Y.-M.; Gwo, S. Large-Scale Hot Spot Engineering for Quantitative SERS at the Single-Molecule Scale. J. Am. Chem. Soc. 2015, 137, 13698–13705. [Google Scholar] [CrossRef]
- Huang, J.-A.; Zhao, Y.-Q.; Zhang, X.-J.; He, L.-F.; Wong, T.-L.; Chui, Y.-S.; Zhang, W.-J.; Lee, S.-T. Ordered Ag/Si Nanowires Array: Wide-Range Surface-Enhanced Raman Spectroscopy for Reproducible Biomolecule Detection. Nano Lett. 2013, 13, 5039–5045. [Google Scholar] [CrossRef]
- Peksa, V.; Lebruskova, P.; Sipova, H.; Stepanek, J.; Bok, J.; Homola, J.; Prochazka, M. Testing gold nanostructures fabricated by hole-mask colloidal lithography as potential substrates for SERS sensors: Sensitivity, signal variability, and the aspect of adsorbate deposition. Phys. Chem. Chem. Phys. 2016, 18, 19613–19620. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, L.; Dai, Z.; Liu, J.; Yang, S.; Zhou, L.; Xiao, X.; Jiang, C.; Roy, V.A.L. Low-Cost, Disposable, Flexible and Highly Reproducible Screen Printed SERS Substrates for the Detection of Various Chemicals (vol 5, 10208, 2015). Sci. Rep. 2015, 5, 12205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamil, A.K.M.; Izake, E.L.; Sivanesan, A.; Agoston, R.; Ayoko, G.A. A homogeneous surface-enhanced Raman scattering platform for ultra-trace detection of trinitrotoluene in the environment. Anal. Methods 2015, 7, 3863–3868. [Google Scholar] [CrossRef] [Green Version]
- Fu, Q.; Zhan, Z.; Dou, J.; Zheng, X.; Xu, R.; Wu, M.; Lei, Y. Highly Reproducible and Sensitive SERS Substrates with Ag Inter-Nanoparticle Gaps of 5 nm Fabricated by Ultrathin Aluminum Mask Technique. ACS Appl. Mater. Interfaces 2015, 7, 13322–13328. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, M.; Sambathkumar, K.; Jeyavijayan, S. Density functional theory study of FTIR and FT-Raman spectra of 7-acetoxy-4-methyl coumarin. Indian J. Pure Appl. Phys. 2010, 48, 716–722. [Google Scholar]
- Evaluation of Measurement Data—Supplement 1 to the “Guide to the Expression of Uncertainty in Measurement”—Propagation of Distributions Using a Monte Carlo Method; JCGM: Paris, France, 2008.







| Intra-Map | ||
|---|---|---|
| Replicate | Mean (I 1595 cm−1) | RSD |
| 1 | 311 | 33% |
| 2 | 398 | 21% |
| 3 | 340 | 27% |
| Inter-map | ||
| Mean (I 1595 cm−1) | RSD | |
| 1, 2, 3 | 350 | 10.3% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandrile, L.; Giovannozzi, A.M.; Sacco, A.; Martra, G.; Rossi, A.M. Flexible and Transparent Substrates Based on Gold Nanoparticles and TiO2 for in Situ Bioanalysis by Surface-Enhanced Raman Spectroscopy. Biosensors 2019, 9, 145. https://0-doi-org.brum.beds.ac.uk/10.3390/bios9040145
Mandrile L, Giovannozzi AM, Sacco A, Martra G, Rossi AM. Flexible and Transparent Substrates Based on Gold Nanoparticles and TiO2 for in Situ Bioanalysis by Surface-Enhanced Raman Spectroscopy. Biosensors. 2019; 9(4):145. https://0-doi-org.brum.beds.ac.uk/10.3390/bios9040145
Chicago/Turabian StyleMandrile, Luisa, Andrea Mario Giovannozzi, Alessio Sacco, Gianmario Martra, and Andrea Mario Rossi. 2019. "Flexible and Transparent Substrates Based on Gold Nanoparticles and TiO2 for in Situ Bioanalysis by Surface-Enhanced Raman Spectroscopy" Biosensors 9, no. 4: 145. https://0-doi-org.brum.beds.ac.uk/10.3390/bios9040145