Activated Porous Carbon Derived from Tea and Plane Tree Leaves Biomass for the Removal of Pharmaceutical Compounds from Wastewaters
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterizations
2.1.1. N2 Physisorption Tests
2.1.2. SEM Images
2.2. Adsorption Evaluation
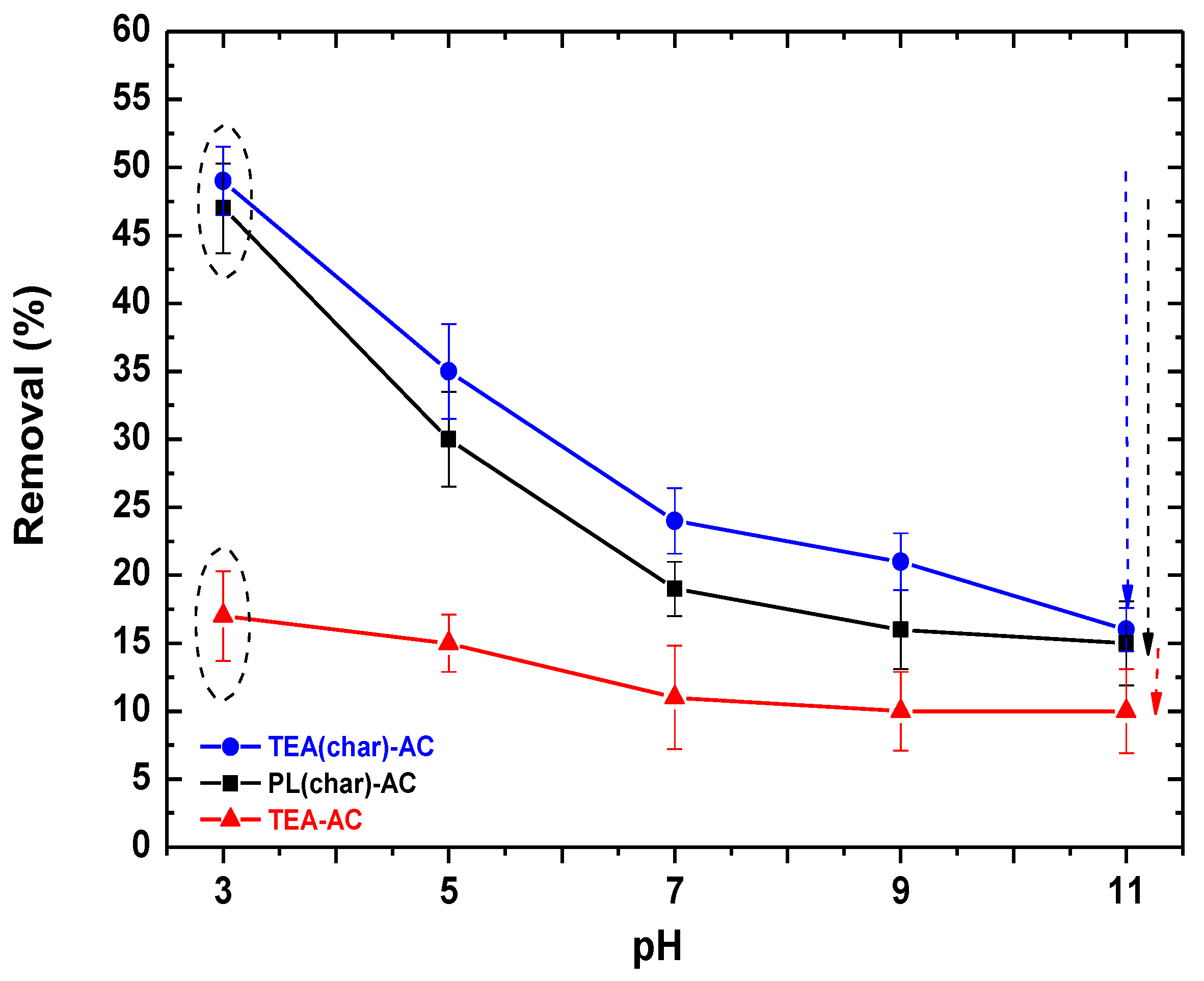
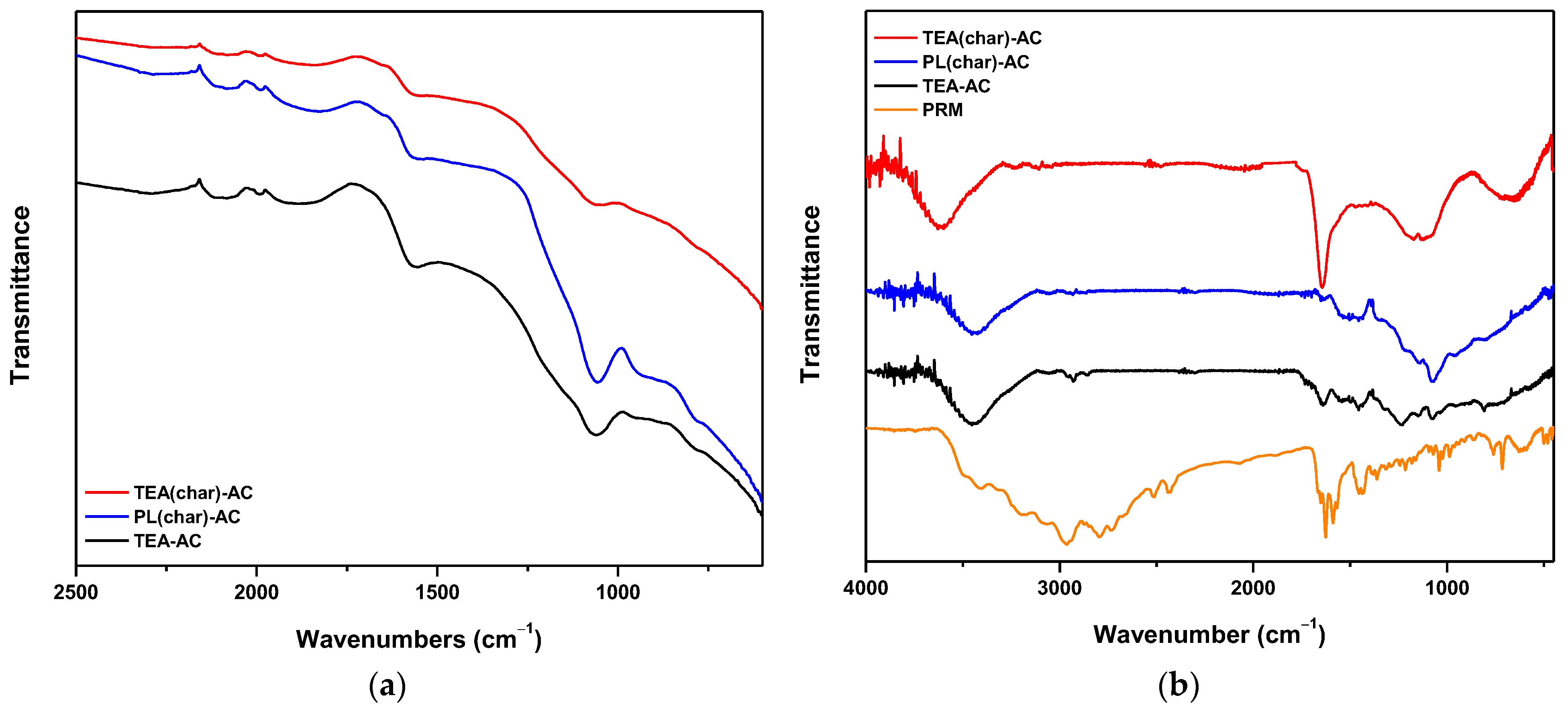
2.2.1. Effect of pH–FTIR Explanations
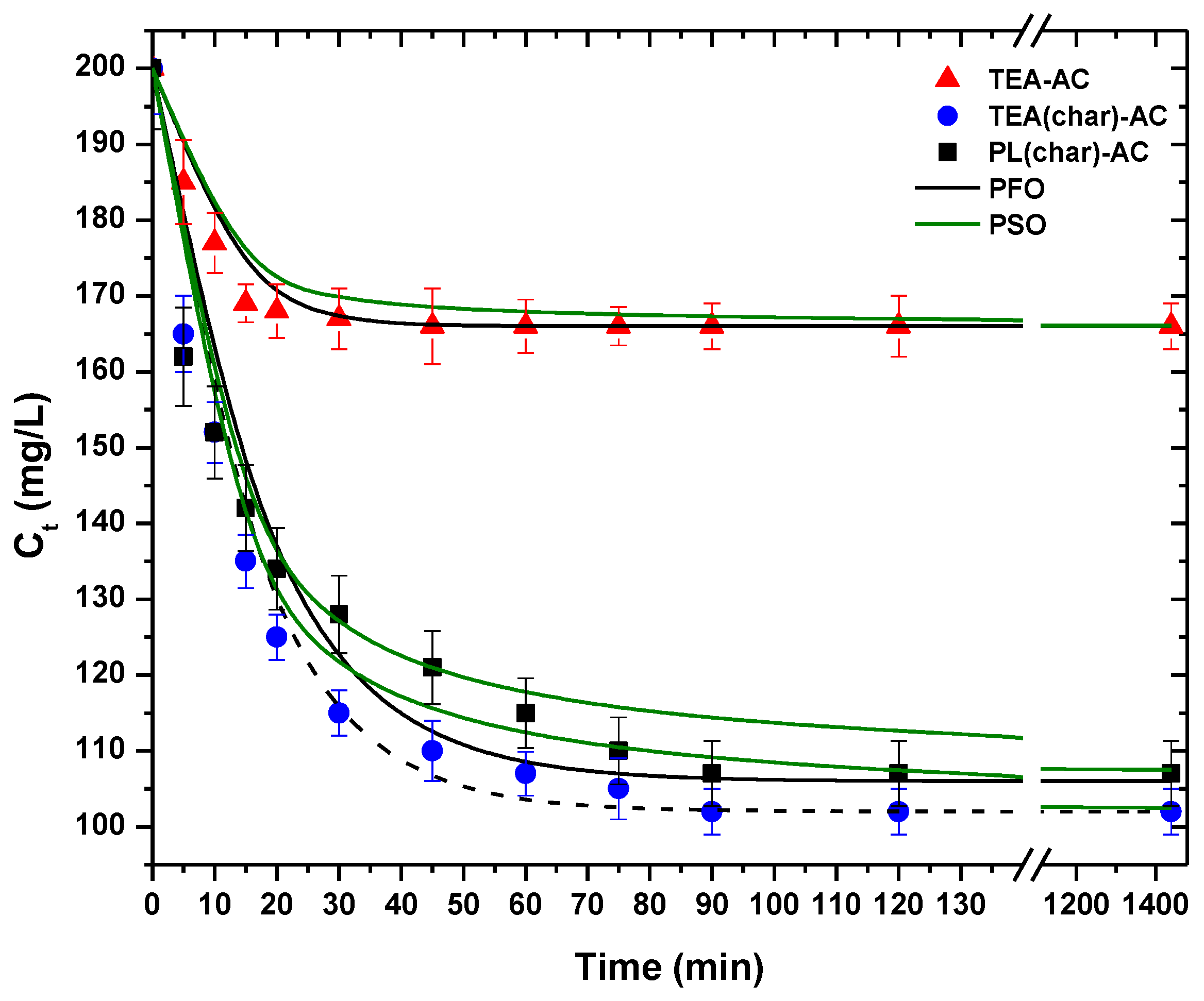
2.2.2. Equilibrium Isotherms and Kinetics
2.3. Comparison with Other Materials
3. Materials and Methods
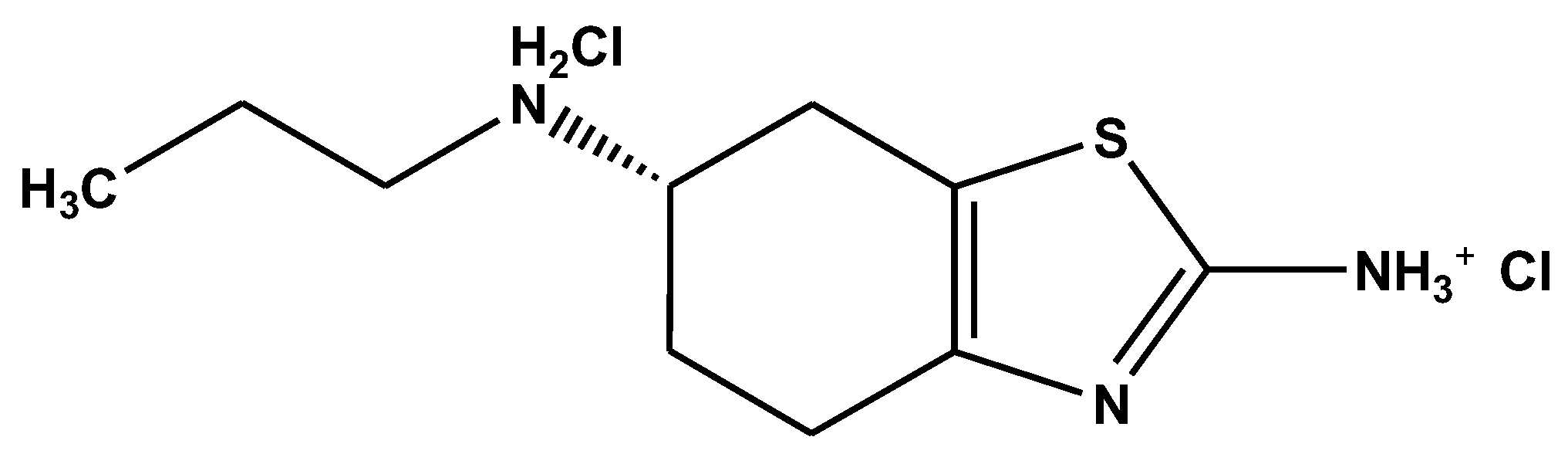
3.1. Materials
3.2. Synthesis of Activated Carbon Samples
3.2.1. PL(char)-AC and TEA(char)-AC
3.2.2. TEA-AC
3.3. Adsorption Evaluation
3.3.1. Effect of the Initial pH
3.3.2. Isotherms
3.3.3. Effect of Contact Time
3.3.4. Analysis and Modeling
3.4. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Cheng, X.; Kyzas, G.Z.; Fu, J. Pharmaceuticals pollution of aquaculture and its management in China. J. Mol. Liq. 2016, 223, 781–789. [Google Scholar] [CrossRef]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ing, A.W.C.; Abdullah, W.R.W.; Hamzah, S.; Azaman, F. Preparation of high-performance adsorbent from low-cost agricultural waste (Peanut husk) using full factorial design: Application to dye removal. Biointerface Res. Appl. Chem. 2020, 10, 6619–6628. [Google Scholar]
- Ali, S.F.A.; Gad, E.S. Investigation of an adsorbent based on novel starch/chitosan nanocomposite in extraction of indigo carmine dye from aqueous solutions. Biointerface Res. Appl. Chem. 2020, 10, 5556–5563. [Google Scholar]
- Batool, M.; Khurshid, S.; Qureshi, Z.M.; Daoush, W.M.; Hashmi, F.; Mehboob, N. Effective adsorptive removal of azodyes on synthesized copper oxide nanoparticles. Biointerface Res. Appl. Chem. 2020, 10, 5369–5375. [Google Scholar]
- Cotrim, A.C.M.; França, E.L.; França, A.C.H.; Martins, J.S.; Silva, K.P.G.; Ghalfi, Y.C.; Machado, I.T.; Tozetti, I.A. Effect of polyethylene glycol microspheres adsorbed with melatonin on oxidative stress and viscosity of cervical mucus samples infected with human papillomavirus. Biointerface Res. Appl. Chem. 2020, 10, 6757–6772. [Google Scholar]
- Kulkarni, S. Synthesis, characterization and performance of low-cost unconventional adsorbents derived from waste materials. Biointerface Res. Appl. Chem. 2020, 10, 7243–7256. [Google Scholar]
- Stackelberg, P.E.; Gibs, J.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Lippincott, R.L. Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci. Total Environ. 2007, 377, 255–272. [Google Scholar] [CrossRef]
- Ekwanzala, M.D.; Lehutso, R.F.; Kasonga, T.K.; Dewar, J.B.; Momba, M.N.B. Environmental dissemination of selected antibiotics from hospital wastewater to the aquatic environment. Antibiotics 2020, 9, 431. [Google Scholar] [CrossRef]
- Piotrowska, M.; Dziewit, L.; Ostrowski, R.; Chmielowska, C.; Popowska, M. Molecular characterization and comparative genomics of incq-3 plasmids conferring resistance to various antibiotics isolated from a wastewater treatment plant in Warsaw (Poland). Antibiotics 2020, 9, 613. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Ilia, A.; Mpekoulis, G.; Papadopoulou, C. Antimicrobial resistance in bacterial pathogens and detection of carbapenemases in klebsiella pneumoniae isolates from hospital wastewater. Antibiotics 2019, 8, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2007, 41, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Gholamirad, F.; Yu, M.; Park, C.M.; Jang, A.; Jang, M.; Taheri-Qazvini, N.; Yoon, Y. Enhanced adsorption performance for selected pharmaceutical compounds by sonicated Ti3C2TX MXene. Chem. Eng. J. 2021, 406, 126789. [Google Scholar] [CrossRef]
- Fu, B.; Ferronato, C.; Fine, L.; Meunier, F.; Luis Valverde, J.; Ferro Fernandez, V.R.; Giroir-Fendler, A.; Chovelon, J.-M. Removal of Pemetrexed from aqueous phase using activated carbons in static mode. Chem. Eng. J. 2021, 405, 127016. [Google Scholar] [CrossRef]
- Sher, F.; Hanif, K.; Rafey, A.; Khalid, U.; Zafar, A.; Ameen, M.; Lima, E.C. Removal of micropollutants from municipal wastewater using different types of activated carbons. J. Environ. Manag. 2021, 278, 111302. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Acharya, R.; Parida, K. A review on adsorptive remediation of cr (Vi) by magnetic iron oxides and their modified forms. Biointerface Res. Appl. Chem. 2020, 10, 5266–5272. [Google Scholar]
- Anuar, F.I.; Hadibarata, T.; Syafrudin, M.; Fona, Z. Removal of procion red MX-5B from aqueous solution by adsorption on parkia speciosa (Stink bean) peel powder. Biointerface Res. Appl. Chem. 2020, 10, 4774–4779. [Google Scholar]
- Kanthasamy, S.; Hadibarata, T.; Hidayat, T.; Alamri, S.A.; Al-Ghamdi, A.A. Adsorption of azo and anthraquinone dye by using watermelon peel powder and corn peel powder: Equilibrium and kinetic studies. Biointerface Res. Appl. Chem. 2020, 10, 4706–4713. [Google Scholar]
- Lau, K.B.K.; Hadibarata, T.; Elwina, E.; Dewi, R.; Alsahli, A.A.; Alaraidh, I.A.; Al-Ghamdi, A.A. Reactive dyes adsorption via citrus hystrix peel powder and zea mays cob powder: Characterization, isotherm and kinetic studies. Biointerface Res. Appl. Chem. 2020, 10, 4803–4810. [Google Scholar]
- Satti, Z.; Akhtar, M.; Mazhar, N.; Khan, S.U.; Ahmed, N.; Yasir, Q.M.; Irshad, M.; Pervaiz, R.; Ahmad, W. Adsorption of cadmium from aqueous solution onto untreated gypsum rock material: Equilibrium and kinetics. Biointerface Res. Appl. Chem. 2020, 11, 10755–10764. [Google Scholar]
- Mestre, A.; Carvalho, A. Nanoporous Carbon Synthesis: An Old Story with Exciting New Chapters; Ghrib, T., Ed.; IntechOpen: London, UK, 2018; p. 34. [Google Scholar]
- Ishchuk, O.P.; Sterner, O.; Ellervik, U.; Manner, S. Simple carbohydrate derivatives diminish the formation of biofilm of the pathogenic yeast candida albicans. Antibiotics 2020, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Primahana, G.; Risdian, C.; Mozef, T.; Sudarman, E.; Köck, M.; Wink, J.; Stadler, M. Nonocarbolines A–E, β-carboline antibiotics produced by the rare actinobacterium nonomuraea sp. From Indonesia. Antibiotics 2020, 9, 126. [Google Scholar] [CrossRef] [Green Version]
- Vagos, M.R.; Gomes, M.; Moreira, J.M.R.; Soares, O.S.G.P.; Pereira, M.F.R.; Mergulhão, F.J. Carbon nanotube/poly(Dimethylsiloxane) composite materials to reduce bacterial adhesion. Antibiotics 2020, 9, 434. [Google Scholar] [CrossRef]
- Yonezawa, K.; Kawaguchi, M.; Kaneuji, A.; Ichiseki, T.; Iinuma, Y.; Kawamura, K.; Shintani, K.; Oda, S.; Taki, M.; Kawahara, N. Evaluation of antibacterial and cytotoxic properties of a fluorinated diamond-like carbon coating for the development of antibacterial medical implants. Antibiotics 2020, 9, 495. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Yi, P.; Zhang, P.; Zhang, L.; Wu, M.; Pan, B. Key roles of electron cloud density and configuration in the adsorption of sulfonamide antibiotics on carbonaceous materials: Molecular dynamics and quantum chemical investigations. Appl. Surf. Sci. 2021, 536, 147757. [Google Scholar] [CrossRef]
- Manjunath, S.V.; Kumar, M. Simultaneous removal of antibiotic and nutrients via Prosopis juliflora activated carbon column: Performance evaluation, effect of operational parameters and breakthrough modeling. Chemosphere 2021, 262, 127820. [Google Scholar] [CrossRef]
- Wang, B.; Xu, X.; Tang, H.; Mao, Y.; Chen, H.; Ji, F. Highly efficient adsorption of three antibiotics from aqueous solutions using glucose-based mesoporous carbon. Appl. Surf. Sci. 2020, 528, 147048. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhu, X.; Wang, J. Adsorptive removal of plasticizer (dimethyl phthalate) and antibiotic (sulfamethazine) from municipal wastewater by magnetic carbon nanotubes. J. Mol. Liq. 2020, 319, 114267. [Google Scholar] [CrossRef]
- Amuda, O.S.; Giwa, A.A.; Bello, I.A. Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem. Eng. J. 2007, 36, 174–181. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, A.; Zhao, Y. Preparation and characterisation of activated carbon from waste tea by physical activation using steam. J. Air Waste Manag. Assoc. 2018, 68, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A. Porous carbons in adsorption and catalysis. In Handbook of Surfaces and Interfaces of Materials; Academic Press: Cambridge, MA, USA, 2001; pp. 309–355. [Google Scholar]
- Üner, O.; Bayrak, Y. The effect of carbonization temperature, carbonization time and impregnation ratio on the properties of activated carbon produced from Arundo donax. Microporous Mesoporous Mater. 2018, 268, 225–234. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crops Prod. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the BET equation applicable to microporous adsorbents. Stud. Surf. Sci. Catal 2007, 160, 1016. [Google Scholar]
- Li, W.; Zhang, L.-B.; Peng, J.-H.; Li, N.; Zhu, X.-Y. Preparation of high surface area activated carbons from tobacco stems with K2CO3 activation using microwave radiation. Ind. Crop. Prod. 2008, 27, 341–347. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A. Modified activated carbons from potato peels as green environmental-friendly adsorbents for the treatment of pharmaceutical effluents. Chem. Eng. Res. Des. 2015, 97, 135–144. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A.; Lazaridis, N.K. Magnetic modification of microporous carbon for dye adsorption. J. Colloid Interface Sci. 2014, 430, 166–173. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Oxidation time effect of activated carbons for drug adsorption. Chem. Eng. J. 2013, 234, 491–499. [Google Scholar] [CrossRef]
- Geczo, A.; Giannakoudakis, D.A.; Triantafyllidis, K.; Elshaer, M.R.; Rodríguez-Aguado, E.; Bashkova, S. Mechanistic insights into acetaminophen removal on cashew nut shell biomass-derived activated carbons. Environ. Sci. Pollut. Res. 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Giannakoudakis, D.A.; Barczak, M.; Florent, M.; Bandosz, T.J. Analysis of interactions of mustard gas surrogate vapors with porous carbon textiles. Chem. Eng. J. 2019, 362, 758–766. [Google Scholar] [CrossRef]
- Kampouraki, Z.C.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Deliyanni, E.A. Catalytic oxidative desulfurization of a 4,6-DMDBT containing model fuel by metal-free activated carbons: The key role of surface chemistry. Green Chem. 2019, 21, 6685–6698. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding chemical reactions between carbons and NaOH and KOH: An insight into the chemical activation mechanism. Carbon 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-activated carbon of high surface area produced from coconut shell: Kinetics and equilibrium studies from the methylene blue adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Le Van, K.; Thu, T.L.T.; Thu, H.N.T.; Van Hoang, H. Activated Carbon by KOH and NaOH Activation: Preparation and Electrochemical Performance in K2SO4 and Na2SO4 Electrolytes. Russ. J. Electrochem. 2019, 55, 900–907. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of Sulfonamide Antibiotics to Multiwalled Carbon Nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Kostoglou, M.; Lazaridis, N.K.; Lambropoulou, D.A.; Bikiaris, D.N. Environmental friendly technology for the removal of pharmaceutical contaminants from wastewaters using modified chitosan adsorbents. Chem. Eng. J. 2013, 222, 248–258. [Google Scholar] [CrossRef]
- Hussain, S.; Anjali, K.P.; Hassan, S.T.; Dwivedi, P.B. Waste tea as a novel adsorbent: A review. Appl. Water Sci. 2018, 8, 165. [Google Scholar] [CrossRef] [Green Version]
- Güler, Z.; Dursun, A.; Özkan, D. Volatile compounds in the Leaf of Plane Tree (Platanus orientalis) with Solid Phase Microextraction (SPME) Technique. Int. J. Second. Metab. 2017, 4, 167–176. [Google Scholar]
- Pourkhabbaz, A.; Rastin, N.; Olbrich, A.; Langenfeld-Heyser, R.; Polle, A. Influence of environmental pollution on Leaf properties of urban plane trees, Platanus orientalis L. Bull. Environ. Contam. Toxicol. 2010, 85, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Hubble, J.P.; Novak, P. Pramipexole: A nonergot dopamine agonist as drug therapy in Parkinson’s disease. Expert Rev. Neurother. 2001, 1, 43–51. [Google Scholar] [CrossRef]
- McCormack, P.L.; Siddiqui, M.A.A. Pramipexole: In restless legs syndrome. CNS Drugs 2007, 21, 429–437. [Google Scholar] [CrossRef]
- Hollingworth, S.A.; Rush, A.; Hall, W.D.; Eadie, M.J. Utilization of anti-Parkinson drugs in Australia: 1995–2009. Pharmacoepidemiol. Drug Saf. 2011, 20, 450–456. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Over the adsorption in solution. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 451–465. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazardous Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, Y.-S. Second-order Kinetic Model for the Sorption of Cadmium onto Tree Fern: A Comparison of Linear and Non-linear Methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput. Methods Programs Biomed. 2001, 65, 191–200. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef]
- Gimbert, F.; Morin-Crini, N.; Renault, F.; Badot, P.M.; Crini, G. Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: Error analysis. J. Hazard. Mater. 2008, 157, 34–46. [Google Scholar] [CrossRef]
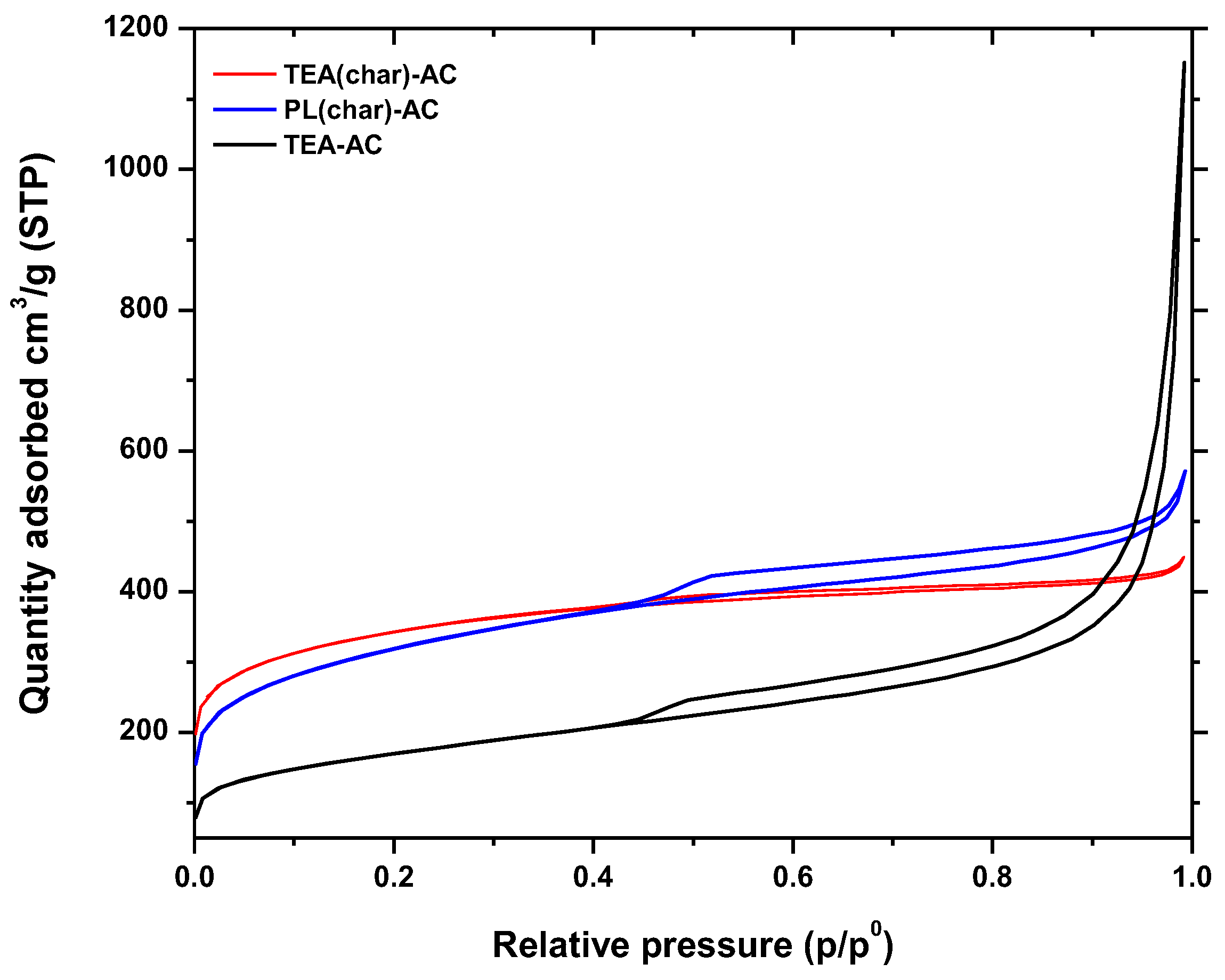
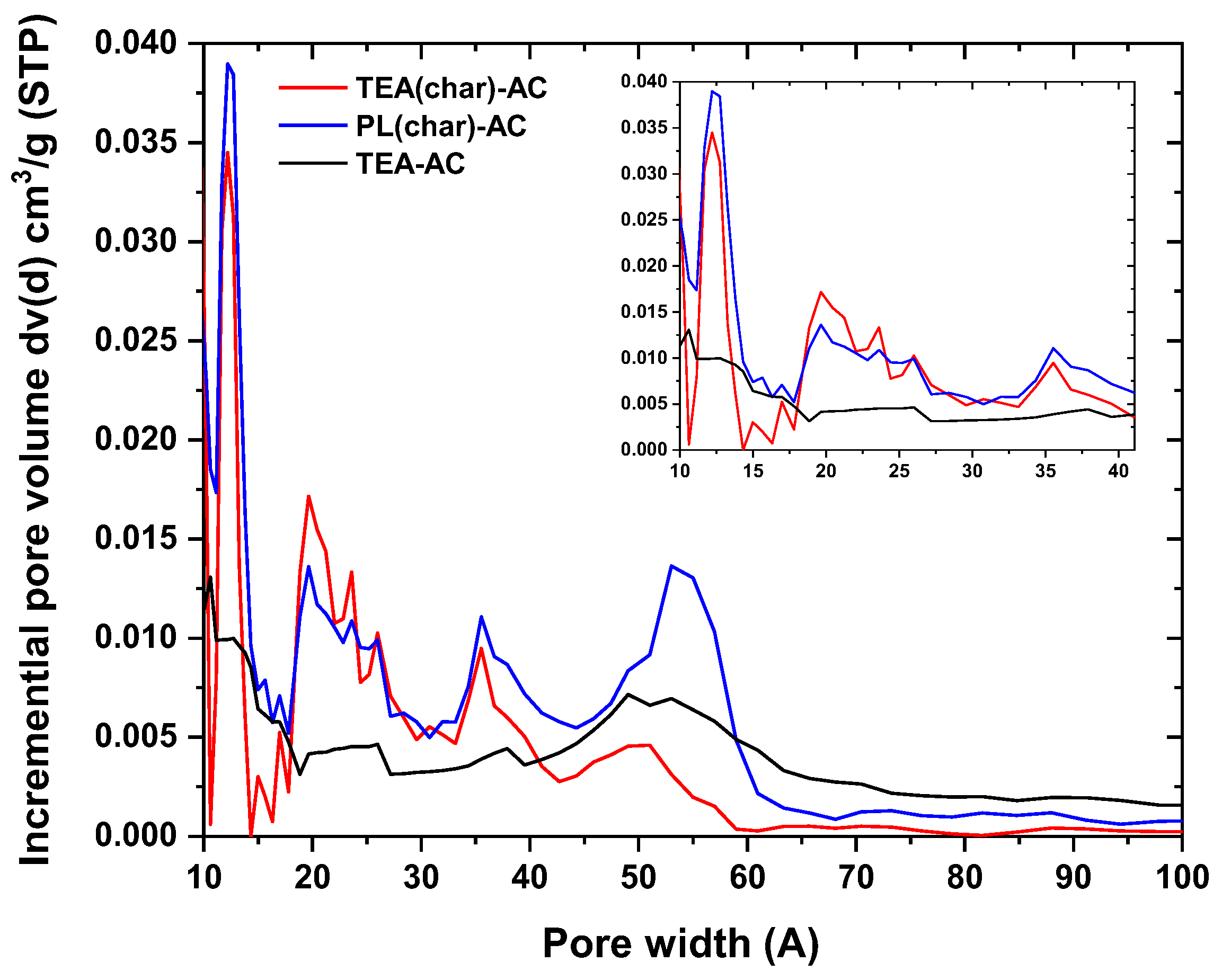



| Parameters | TEA(char)-AC | PL(char)-AC | TEA-AC |
|---|---|---|---|
| Surface area (m2/g) | 1151 | 1105 | 594 |
| Micropore surface area (m2/g) | 774 | 508 | 168 |
| External surface area (m2/g) | 376.7 | 596.6 | 425.7 |
| Total Pore volume (cm3/g) | 0.694 | 0.884 | 1.782 |
| Micropore volume (cm3/g) | 0.369 | 0.236 | 0.078 |
| Mesopore volume(cm3/g) | 0.325 | 0.649 | 1.703 |
| Ratio of micro- to mesopores volume | 1.14 | 0.36 | 0.05 |
| Langmuir Equation | Freundlich Equation | |||||
|---|---|---|---|---|---|---|
| Qm | KL | R2 | KF | n | R2 | |
| Material | mg/g | L/mg | mg1−1/n L1/n g−1 | |||
| TEA(char)-AC | 112 | 0.091 | 0.969 | 23.19 | 3.085 | 0.847 |
| PL(char)-AC | 101 | 0.063 | 0.962 | 18.16 | 2.763 | 0.846 |
| TEA-AC | 34 | 0.004 | 0.917 | 0.83 | 1.383 | 0.952 |
| Pseudo First-Order | Pseudo Second-Order | |||
|---|---|---|---|---|
| k1 | R2 | k2 | R2 | |
| Material | (min−1) | (g mg−1 min−1) | ||
| TEA(char)-AC | 0.1255 | 0.994 | 0.1422 | 0.970 |
| PL(char)-AC | 0.0622 | 0.963 | 0.1294 | 0.980 |
| TEA-AC | 0.0713 | 0.994 | 0.2838 | 0.946 |
| Adsorbent | Qmax (mg/g) | Reference |
|---|---|---|
| Sulfonate grafted chitosan | 337 | [53] |
| N-(2-carboxybenzyl) grafted chitosan | 307 | [53] |
| Chitosan | 181 | [53] |
| Activated carbons (BAX) | 117 | [45] |
| Activated carbon from potato peels (hydrothermal) | 66 | [43] |
| Activated carbon from potato peels (pyrolized) | 56 | [43] |
| TEA(char)-AC | 112 | This study |
| PL(char)-AC | 101 | This study |
| TEA-AC | 34 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liakos, E.V.; Rekos, K.; Giannakoudakis, D.A.; Mitropoulos, A.C.; Fu, J.; Kyzas, G.Z. Activated Porous Carbon Derived from Tea and Plane Tree Leaves Biomass for the Removal of Pharmaceutical Compounds from Wastewaters. Antibiotics 2021, 10, 65. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010065
Liakos EV, Rekos K, Giannakoudakis DA, Mitropoulos AC, Fu J, Kyzas GZ. Activated Porous Carbon Derived from Tea and Plane Tree Leaves Biomass for the Removal of Pharmaceutical Compounds from Wastewaters. Antibiotics. 2021; 10(1):65. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010065
Chicago/Turabian StyleLiakos, Efstathios V., Kyriazis Rekos, Dimitrios A. Giannakoudakis, Athanasios C. Mitropoulos, Jie Fu, and George Z. Kyzas. 2021. "Activated Porous Carbon Derived from Tea and Plane Tree Leaves Biomass for the Removal of Pharmaceutical Compounds from Wastewaters" Antibiotics 10, no. 1: 65. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010065