Ceftolozane-Tazobactam Combination Therapy Compared to Ceftolozane-Tazobactam Monotherapy for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Search
2.2. Study Selection
2.3. Definition and Outcome
2.4. Data Extraction and Quality Assessment
2.5. Data Analysis
3. Results
3.1. Study Selection and Characteristics
3.2. Quantitative Synthesis
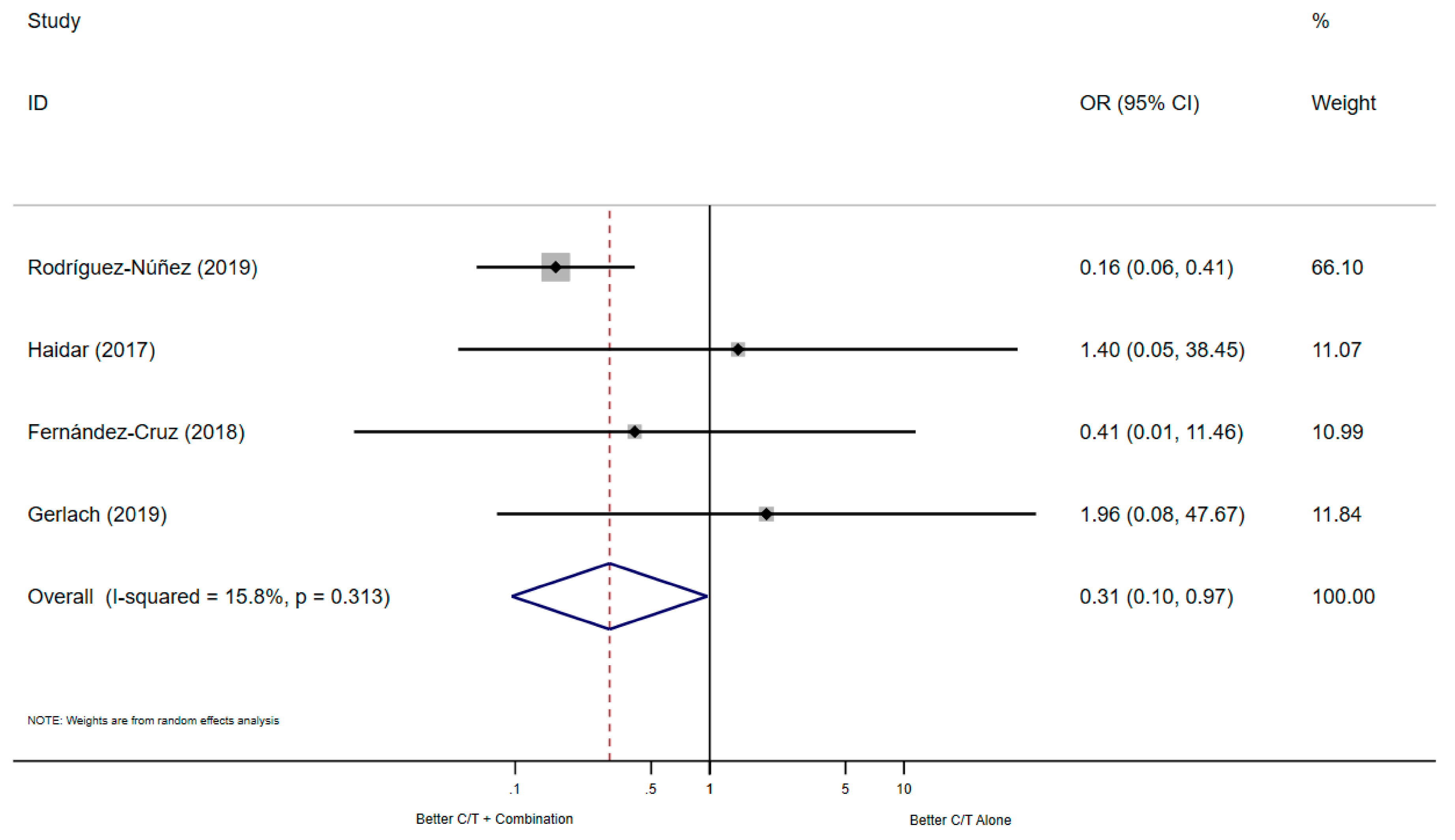
3.2.1. All-Cause Mortality
3.2.2. Clinical Improvement
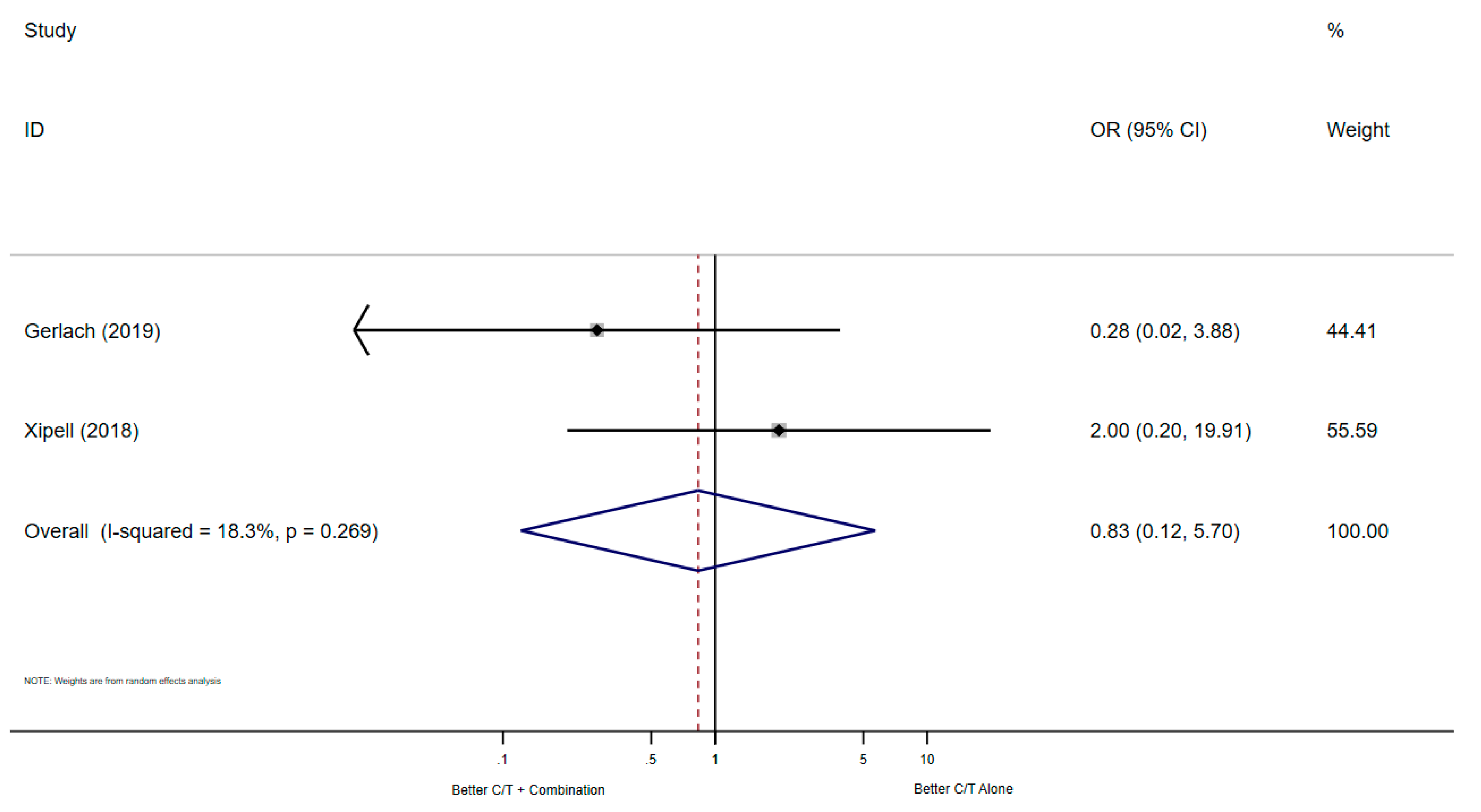
3.2.3. Microbiological Cure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zerbaxa (accessed on 3 December 2020).
- Food and Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206829lbl.pdf (accessed on 3 December 2020).
- Farrell, D.J.; Flamm, R.K.; Sader, H.S.; Jones, R.N. Antimicrobial activity of ceftolozane-tazobactam tested against Enterobacteriaceae and Pseudomonas aeruginosa with various resistance patterns isolated in U.S. Hospitals (2011–2012). Antimicrob. Agents Chemother. 2013, 57, 6305–6310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rico Caballero, V.; Almarzoky Abuhussain, S.; Kuti, J.L.; Nicolau, D.P. Efficacy of Human-Simulated Exposures of Ceftolozane-Tazobactam Alone and in Combination with Amikacin or Colistin against Multidrug-Resistant Pseudomonas aeruginosa in an In Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2018, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galani, I.; Papoutsaki, V.; Karantani, I.; Karaiskos, I.; Galani, L.; Adamou, P.; Deliolanis, I.; Kodonaki, A.; Papadogeorgaki, E.; Markopoulou, M.; et al. In vitro activity of ceftolozane/tazobactam alone and in combination with amikacin against MDR/XDR Pseudomonas aeruginosa isolates from Greece. J. Antimicrob. Chemother. 2020, 75, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Dassner, A.M.; Sutherland, C.; Girotto, J.; Nicolau, D.P. In vitro Activity of Ceftolozane/Tazobactam Alone or with an Aminoglycoside Against Multi-Drug-Resistant Pseudomonas aeruginosa from Pediatric Cystic Fibrosis Patients. Infect. Dis. Ther. 2017, 6, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuba, G.T.; Rocha-Santos, G.; Cayô, R.; Streling, A.P.; Nodari, C.S.; Gales, A.C.; Pignatari, A.C.C.; Nicolau, D.P.; Kiffer, C.R.V. In vitro synergy of ceftolozane/tazobactam in combination with fosfomycin or aztreonam against MDR Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020, 75, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.J.O.O.H.R.I. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011. [Google Scholar]
- Haidar, G.; Philips, N.J.; Shields, R.K.; Snyder, D.; Cheng, S.; Potoski, B.A.; Doi, Y.; Hao, B.; Press, E.G.; Cooper, V.S.; et al. Ceftolozane-Tazobactam for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Infections: Clinical Effectiveness and Evolution of Resistance. Clin. Infect. Dis. 2017, 65, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cruz, A.; Alba, N.; Semiglia-Chong, M.A.; Padilla, B.; Rodríguez-Macías, G.; Kwon, M.; Cercenado, E.; Chamorro-de-Vega, E.; Machado, M.; Pérez-Lago, L.; et al. A Case-Control Study of Real-Life Experience with Ceftolozane-Tazobactam in Patients with Hematologic Malignancy and Pseudomonas aeruginosa Infection. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xipell, M.; Paredes, S.; Fresco, L.; Bodro, M.; Marco, F.; Martínez, J.A.; Soriano, A. Clinical experience with ceftolozane/tazobactam in patients with serious infections due to resistant Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist 2018, 13, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Castaldo, N.; Cattelan, A.; Mussini, C.; Righi, E.; Tascini, C.; Menichetti, F.; Mastroianni, C.M.; Tumbarello, M.; Grossi, P.; et al. Ceftolozane/tazobactam for the treatment of serious Pseudomonas aeruginosa infections: A multicentre nationwide clinical experience. Int. J. Antimicrob. Agents 2019, 53, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cañestro, M.; Periañez, L.; Mulet, X.; Martin-Pena, M.L.; Fraile-Ribot, P.A.; Ayestarán, I.; Colomar, A.; Nuñez, B.; Maciá, M.; Novo, A.; et al. Ceftolozane/tazobactam for the treatment of multidrug resistant Pseudomonas aeruginosa: Experience from the Balearic Islands. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2191–2200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, O.; Periañez-Parraga, L.; Oliver, A.; Munita, J.M.; Boté, A.; Gasch, O.; Nuvials, X.; Dinh, A.; Shaw, R.; Lomas, J.M.; et al. Higher MICs (>2mg/L) Predict 30-Day Mortality in Patients With Lower Respiratory Tract Infections Caused by Multidrug- and Extensively Drug-Resistant Pseudomonas aeruginosa Treated With Ceftolozane/Tazobactam. Open Forum. Infect. Dis. 2019, 6, ofz416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlach, A.T.; Goff, D.A.; Bazan, J.A. Ceftolozane/Tazobactam for the Treatment of Osteomyelitis Due to Multidrug-Resistant Pseudomonas aeruginosa. Infect. Dis. Clin. Pract. 2019, 27, 339–342. [Google Scholar] [CrossRef]
- Bassetti, M.; Vena, A.; Giacobbe, D.R.; Falcone, M.; Tiseo, G.; Giannella, M.; Pascale, R.; Meschiari, M.; Digaetano, M.; Oliva, A.; et al. Ceftolozane/Tazobactam for Treatment of Severe ESBL-Producing Enterobacterales Infections: A Multicenter Nationwide Clinical Experience (CEFTABUSE II Study). Open Forum. Infect. Dis. 2020, 7, ofaa139. [Google Scholar] [CrossRef] [PubMed]
- Infectious Diseases Society of, A.; Spellberg, B.; Blaser, M.; Guidos, R.J.; Boucher, H.W.; Bradley, J.S.; Eisenstein, B.I.; Gerding, D.; Lynfield, R.; Reller, L.B.; et al. Combating antimicrobial resistance: Policy recommendations to save lives. Clin. Infect. Dis. 2011, 52 (Suppl. 5), S397–S428. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Alfieri, A.; Di Franco, S.; Pace, M.C.; Simeon, V.; Ingoglia, G.; Cortegiani, A. Ceftazidime-Avibactam Combination Therapy Compared to Ceftazidime-Avibactam Monotherapy for the Treatment of Severe Infections Due to Carbapenem-Resistant Pathogens: A Systematic Review and Network Meta-Analysis. Antibiotics 2020, 9, 388. [Google Scholar] [CrossRef] [PubMed]


| Participants | Intervention | Comparison | Outcomes | Study Design |
|---|---|---|---|---|
| Adult patients in any setting with microbiological confirmed bacterial infection | Ceftolozane-tazobactam in association with another antibiotic/s | Ceftolozane-tazobactam alone | Primary outcomes: All-cause mortality Secondary outcomes: (a) Clinical improvement (b) Microbiological cure | Randomized controlled trials and observational Studies (including cohort and case–control studies) |
| Author (Published Year) [Ref.] | Journal | Study Design | Country | Time Span | Pathogen | Septic Focus | Evaluation Time Points | ||
|---|---|---|---|---|---|---|---|---|---|
| Mortality | Clinical | Microbiological | |||||||
| Haidar (2017) [9] | Clinical Infectious Diseases | A single-center Retrospective cohort study | USA | 06/15–03/16 | MDR-PA | MIX | 30-days | 90-days | - |
| Fernández-Cruz (2018) [10] | Antimicrobial Agents and Chemotherapy | A single-center case-control study | Spain | 03/16–02/18 | PA | MIX | 30-days | 14-days | - |
| Xipell (2018) [11] | Journal of Global Antimicrobial Resistance | A single-center Retrospective cohort study | Spain | 05/16–05/17 | PA | MIX | - | NA | after 72 h of treatment |
| Bassetti (2018) [12] | International Journal of Antimicrobial Agents | Multicenter Retrospective cohort study | Italy | 06/16–03/18 | PA | MIX | - | MIX (7–23 M) | - |
| Díaz-Cañestro (2018) [13] | European Journal of Clinical Microbiology and Infectious Diseases | A single-center Retrospective cohort study | Spain | 05/16–09/17 | MDR/XDR-PA | MIX | - | after 7 days of treatment | - |
| Rodríguez-Núñez (2019) [14] | Open Forum Infectious Diseases | Multicentre Retrospective cohort study | USA France Spain UK | 2016–2018 | MDR/XDR-PA | LRI | 30-days | - | - |
| Gerlach (2019) [15] | Infectious Diseases in Clinical Practice | A single-center Retrospective cohort study | USA | 06/15–10/17 | PA | Osteomyelitis | 30-days | End-of-Therapy | any follow-up |
| Bassetti (2020) [16] | Open Forum Infectious Diseases | Multicentre Retrospective cohort study | Italy | 06/2016–06/2019 | ESBL | MIX | ! | At the end of the follow-up period (August 2019) | - |
| Author (Published Year) [Ref.] | Country | No. of Patients Enrolled | No. of Patients Treated with C/T Alone | No. of Patients Treated with C/T Association | No. of Patients Treated with BAT | BAT | C/T-Associated Antibiotic | Medical Ward |
|---|---|---|---|---|---|---|---|---|
| Haidar (2017) [9] | USA | 21 | 19 | 2 | X | X | + | NS |
| Fernández-Cruz (2018) [10] | Spain | 57 | 11 | 8 | 38 | ¥ | & | Hematological ward + Hematopoietic Stem Cell Transplantation Unit ICU: 12 (21.1%) |
| Rodríguez-Núñez (2019) [14] | USA France Spain UK | 90 | 54 | 36 | X | X | # | ICU (patients with LRI) |
| Gerlach (2019) [15] | USA | 18 | 3 | 15 | X | X | $ | MIX ICU: 11 (61.1%) |
| Author (Published Year) [Ref.] | Country | No. of Patients Enrolled | No. of Patients Treated with C/T Alone | No. of Patients Treated with C/T Association | No. of Patients Treated with BAT | BAT | C/T-Associated Antibiotic | Medical Ward |
|---|---|---|---|---|---|---|---|---|
| Haidar (2017) [9] | USA | 21 | 19 | 2 | X | X | + | NS |
| Díaz-Cañestro (2018) [13] | Spain | 58 | 21 | 35 | X | X | ^^ | MIX ICU: 16 (27.6%) |
| Bassetti (2018) [12] | Italy | 101 | 65 | 36 | X | X | £ | MIX |
| Fernández-Cruz (2018) [10] | Spain | 57 | 11 | 8 | 38 | ¥ | & | Hematological ward + Hematopoietic Stem Cell Transplantation Unit ICU: 12 (21.1%) |
| Xipell (2018) [11] | Spain | 24 | 15 | 9 | X | X | <> | NS |
| Gerlach (2019) [15] | USA | 18 | 3 | 14 | X | X | $ | MIX ICU: 11 (61.1%) |
| Bassetti (2020) [16] | Italy | 153 | 127 | 26 | X | X | NS | MIX ICU: 30 (19.6%) |
| Author (Published Year) [Ref.] | Country | No. of Patients Enrolled | No. of Patients Treated with C/T Alone | No. of Patients Treated with C/T Association | No. of Patients Treated with BAT | BAT | C/T-Associated Antibiotic | Medical Ward |
|---|---|---|---|---|---|---|---|---|
| Xipell (2018) [11] | Spain | 24 | 10 | 6 | X | X | <> | NS |
| Gerlach (2019) [15] | USA | 18 | 3 | 14 | X | X | $ | MIX ICU: 11 (61.1%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, M.; Corrente, A.; Pace, M.C.; Alfieri, A.; Simeon, V.; Ippolito, M.; Giarratano, A.; Cortegiani, A. Ceftolozane-Tazobactam Combination Therapy Compared to Ceftolozane-Tazobactam Monotherapy for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 79. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010079
Fiore M, Corrente A, Pace MC, Alfieri A, Simeon V, Ippolito M, Giarratano A, Cortegiani A. Ceftolozane-Tazobactam Combination Therapy Compared to Ceftolozane-Tazobactam Monotherapy for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis. Antibiotics. 2021; 10(1):79. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010079
Chicago/Turabian StyleFiore, Marco, Antonio Corrente, Maria Caterina Pace, Aniello Alfieri, Vittorio Simeon, Mariachiara Ippolito, Antonino Giarratano, and Andrea Cortegiani. 2021. "Ceftolozane-Tazobactam Combination Therapy Compared to Ceftolozane-Tazobactam Monotherapy for the Treatment of Severe Infections: A Systematic Review and Meta-Analysis" Antibiotics 10, no. 1: 79. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010079






