The Prospect of Repurposing Immunomodulatory Drugs for Adjunctive Chemotherapy against Tuberculosis: A Critical Review
Abstract
:1. Tuberculosis: The Disease, the Immunobiology and the Available Treatment
2. The Prospect of Repurposing
3. Repurposing Immunomodulatory Compounds
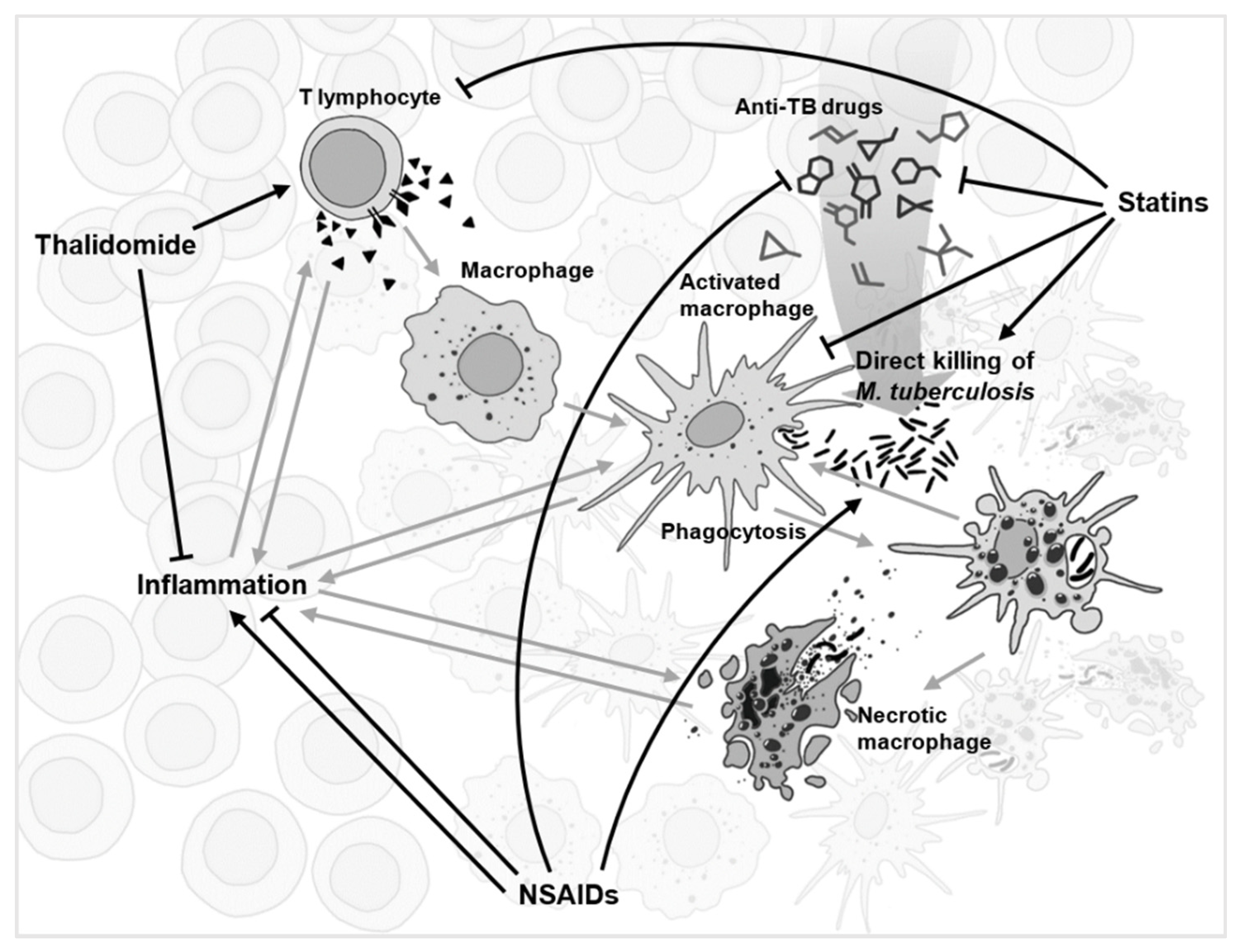
3.1. Immunomodulation Is Diverse
3.2. Anti-Cancer Drugs
3.3. Non-Steroidal Anti-Inflammatory Drugs
| Drug Class | Name | Original Indication | Delivery Route | FDA Approval | Clinical Trial Progression | Availability | Key References |
|---|---|---|---|---|---|---|---|
| Biologic | Denileukin diftitox | Cutaneous T cell lymphoma | IV | 1999 | -- | PO | [68] |
| CCB | Verapamil | Angina | Oral | 1981 | -- | PO | [46,47] |
| HDACi | Phenylbutyrate | Urea cycle disorders | Oral | 1996 | NCT01580007 Phase II trial completed 2018 | PO | [38,39,40] |
| HDACi | Valproic acid | Epilepsy | Oral | 1978 | -- | PO | [39] |
| HDACi | Vorinostat | Cutaneous T cell lymphoma | Oral | 2006 | -- | PO | [39] |
| LOi | -- | Asthma, neoplasms, arthritis | -- | -- | -- | -- | [33,72] |
| NSAID | Aspirin | Arthritis, analgesic | Oral | 1950 | NCT02237365 Phase II trial awaiting results | OTC | [43,82,83,84] |
| NSAID | Carprofen | Analgesic | Oral | 1987 | -- | VUO | [78,88] |
| NSAID | Celecoxib | Arthritis | Oral | 1998 | NCT02602509 Phase I trial completed | PO | [33,81] |
| NSAID | Etoricoxib | Inflammatory disorders | Oral | -- | NCT02503839 Phase I trial underway | PO | [29,33] |
| NSAID | Ibuprofen | Arthritis, analgesic | Oral | 1974 | NCT02781909 Phase II trial underway | OTC | [79,80,81,82] |
| NSAID | Meloxicam | Arthritis | Oral | 2000 | NCT02060006 Phase III trial awaiting results | PO | [33] |
| PDEi | Cilostazol | Cardiovascular disorders | Oral | 1999 | -- | PO | [57,58,59,60,61] |
| PDEi | Sildenafil | Erectile dysfunction | Oral | 1998 | -- | PO | [57,58,59,60,61] |
| Statins | Pravastatin | Cardiovascular disorders | Oral | 1991 | NCT04504851 Phase II trial underway | PO | [49] |
| Statins | Rosuvastatin | Cardiovascular disorders | Oral | 2003 | NCT04504851 Phase II trial in planning | PO | [50] |
| TC | Doxycycline | Bacterial infections | Oral | 1967 | NCT02774993 Phase II trial awaiting results | PO | [44,45] |
| TNFi | -- | Autoimmune disorders | -- | -- | -- | -- | [8] |
| TKI | Fostamatinib | Chronic immune thrombocytopenia | Oral | 2018 | -- | PO | [65,66,67] |
| TKI | Gefitinib | Metastatic non-small cell lung cancer | Oral | 2003 | -- | PO | [65,66,67] |
| TKI | Imatinib | Chronic myeloid leukaemia | Oral | 2001 | NCT03891901 Phase II trial in planning | PO | [62,63] |
| TKI | Nilotinib | Chronic myeloid leukaemia | Oral | 2007 | -- | PO | [65,66,67] |
| Vitamin | Vitamin D | Vitamin | Oral | -- | Several clinical trials completed | OTC | [31,32,33,34,35] |
| -- | Auranofin | Rheumatoid arthritis | Oral | 1985 | NCT02968927 Phase II trial awaiting results | PO | [93] |
| -- | Diosmin | Haemorrhoids | Oral 1 | -- | -- | -- | [71] |
| -- | Isoprinosine | Viral infections | Oral | -- | -- | -- | [73] |
| -- | Thalidomide | Leprosy | Oral | 1998 | Several clinical trials completed | PO | [38,39,40] |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report. 2020. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 9 December 2020).
- Maitra, A.; Munshi, T.; Healy, J.; Martin, L.T.; Vollmer, W.; Keep, N.H.; Bhakta, S. Cell wall peptidoglycan in Mycobacterium tuberculosis: An Achilles’ heel for the TB-causing pathogen. FEMS Microbiol. Rev. 2019, 43, 548–575. [Google Scholar] [CrossRef] [Green Version]
- Sambandan, D.; Dao, D.N.; Weinrick, B.C.; Vilchèze, C.; Gurcha, S.S.; Ojha, A.; Kremer, L.; Besra, G.S.; Hatfull, G.F.; Jacobs, W.R. Keto-Mycolic acid-dependent pellicle formation confers tolerance to drug-sensitive Mycobacterium tuberculosis. MBio 2013, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Machado, D.; Coelho, T.S.; Perdigão, J.; Pereira, C.; Couto, I.; Portugal, I.; Maschmann, R.D.A.; Ramos, D.F.; von Groll, A.; Rossetti, M.L.R.; et al. Interplay between mutations and efflux in drug resistant clinical isolates of Mycobacterium tuberculosis. Front. Microbiol. 2017, 8, 711. [Google Scholar] [CrossRef]
- Ferluga, J.; Yasmin, H.; Al-Ahdal, M.N.; Bhakta, S.; Kishore, U. Natural and trained innate immunity against Mycobacterium tuberculosis. Immunobiology 2020, 225, 151951. [Google Scholar] [CrossRef]
- Gupta, A.; Kaul, A.; Tsolaki, A.G.; Kishore, U.; Bhakta, S. Mycobacterium tuberculosis: Immune evasion, latency and reactivation. Immunobiology 2012, 217, 363–374. [Google Scholar] [CrossRef]
- Gengenbacher, M.; Kaufmann, S.H.E. Mycobacterium tuberculosis: Success through dormancy. FEMS Microbiol. Rev. 2012, 36, 514–532. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Fan, W.; Yang, G.; Xu, Z.; Wang, J.; Cheng, Q.; Yu, M. Risk of tuberculosis in patients treated with TNF-α antagonists: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017, 7, e012567. [Google Scholar] [CrossRef] [Green Version]
- Cicchese, J.M.; Dartois, V.; Kirschner, D.E.; Linderman, J.J. Both Pharmacokinetic Variability and Granuloma Heterogeneity Impact the Ability of the First-Line Antibiotics to Sterilize Tuberculosis Granulomas. Front. Pharmacol. 2020, 11, 333. [Google Scholar] [CrossRef] [Green Version]
- Tait, D.R.; Hatherill, M.; Der Meeren, O.; Van Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef]
- de Gijsel, D.; von Reyn, C.F. A Breath of Fresh Air: BCG Prevents Adult Pulmonary Tuberculosis. Int. J. Infect. Dis. 2019, 80, S6–S8. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, R. Bedaquiline: First FDA-approved tuberculosis drug in 40 years. Int. J. Appl. Basic Med. Res. 2013, 3, 1–2. [Google Scholar] [CrossRef]
- da Cunha, B.R.; Fonseca, L.P.; Calado, C.R.C. Antibiotic discovery: Where have we come from, where do we go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.; Zhan, L.; Liu, Z.H.; Pan, Q.; Chen, X.L.; Xiao, Z.; Qin, C.; Zhang, X.L. Identification of pyrvinium pamoate as an anti-tuberculosis agent in vitro and in vivo by SOSA approach amongst known drugs. Emerg. Microbes Infect. 2020, 9, 302–312. [Google Scholar] [CrossRef]
- Gillespie, S.H. The role of moxifloxacin in tuberculosis therapy. Eur. Respir. Rev. 2016, 25, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Pranger, A.D.; van der Werf, T.S.; Kosterink, J.G.W.; Alffenaar, J.W.C. The Role of Fluoroquinolones in the Treatment of Tuberculosis in 2019. Drugs 2019, 79, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.M.; Sloan, D.J. The role of delamanid in the treatment of drug-resistant tuberculosis. Ther. Clin. Risk Manag. 2015, 11, 779–791. [Google Scholar]
- Xiao, S.; Guo, H.; Weiner, W.S.; Maddox, C.; Mao, C.; Gunosewoyo, H.; Pelly, S.; White, L.E.; Rasmussen, L.; Schoenen, F.J.; et al. Revisiting the β-Lactams for Tuberculosis Therapy with a Compound-Compound Synthetic Lethality Approach. Antimicrob. Agents Chemother. 2019, 63, e01319-19. [Google Scholar] [CrossRef]
- Deshpande, D.; Srivastava, S.; Chapagain, M.; Magombedze, G.; Martin, K.R.; Cirrincione, K.N.; Lee, P.S.; Koeuth, T.; Dheda, K.; Gumbo, T. Ceftazidime-avibactam has potent sterilizing activity against highly drug-resistant tuberculosis. Sci. Adv. 2017, 3, e1701102. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, D.; Srivastava, S.; Bendet, P.; Martin, K.R.; Cirrincione, K.N.; Lee, P.S.; Pasipanodya, J.G.; Dheda, K.; Gumbo, T. Antibacterial and sterilizing effect of benzylpenicillin in tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e02232-17. [Google Scholar] [CrossRef] [Green Version]
- Levine, S.R.; Beatty, K.E. Investigating β-lactam drug targets in. bioRxiv 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Huynh, J.; Marais, B.J. Multidrug-resistant tuberculosis infection and disease in children: A review of new and repurposed drugs. Ther. Adv. Infect. Dis. 2019, 6, 1–16. [Google Scholar] [CrossRef]
- Kalani, K.; Chaturvedi, V.; Trivedi, P.; Tondon, S.; Srivastava, S.K. Dihydroartemisinin and its Analogs: A New Class of Antitubercular Agents. Curr. Top. Med. Chem. 2019, 19, 594–599. [Google Scholar] [CrossRef]
- Patel, Y.S.; Mistry, N.; Mehra, S. Repurposing artemisinin as an anti-mycobacterial agent in synergy with rifampicin. Tuberculosis 2019, 115, 146–153. [Google Scholar] [CrossRef]
- Zheng, H.; Williams, J.T.; Aleiwi, B.; Ellsworth, E.; Abramovitch, R.B. Inhibiting Mycobacterium tuberculosis DosRST Signaling by Targeting Response Regulator DNA Binding and Sensor Kinase Heme. ACS Chem. Biol. 2020, 15, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Martini, M.C.; Zhang, T.; Williams, J.T.; Abramovitch, R.B.; Weathers, P.J.; Shell, S.S. Artemisia annua and Artemisia afra extracts exhibit strong bactericidal activity against Mycobacterium tuberculosis. J. Ethnopharmacol. 2020, 262, 113191. [Google Scholar] [CrossRef]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-directed therapies for bacterial and viral infections. Nat. Rev. Drug Discov. 2017, 17, 35–56. [Google Scholar] [CrossRef]
- Arranz-Trullén, J.; Lu, L.; Pulido, D.; Bhakta, S.; Boix, E. Host antimicrobial peptides: The promise of new treatment strategies against tuberculosis. Front. Immunol. 2017, 8, 1499. [Google Scholar] [CrossRef] [Green Version]
- Anastasopoulou, A.; Ziogas, D.C.; Samarkos, M.; Kirkwood, J.M.; Gogas, H. Reactivation of tuberculosis in cancer patients following administration of immune checkpoint inhibitors: Current evidence and clinical practice recommendations. J. Immunother. Cancer 2019, 7, 239. [Google Scholar] [CrossRef] [Green Version]
- Ndlovu, H.; Marakalala, M.J. Granulomas and inflammation: Host-directed therapies for tuberculosis. Front. Immunol. 2016, 7, 434. [Google Scholar] [CrossRef] [Green Version]
- Campbell, G.R.; Spector, S.A. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy 2012, 8, 1523–1525. [Google Scholar] [CrossRef] [Green Version]
- Greenstein, R.J.; Su, L.; Brown, S.T. Vitamins A & D Inhibit the Growth of Mycobacteria in Radiometric Culture. PLoS ONE 2012, 7, e29631. [Google Scholar]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, D.A.; Ganmaa, D.; Wejse, C.; Raqib, R.; Haq, M.A.; Salahuddin, N.; Daley, P.K.; Ralph, A.P.; Ziegler, T.R.; Martineau, A.R. Adjunctive vitamin D in tuberculosis treatment: Meta-analysis of individual participant data. Eur. Respir. J. 2019, 53, 1802003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soeharto, D.A.; Rifai, D.A.; Marsudidjadja, S.; Roekman, A.E.; Assegaf, C.K.; Louisa, M. Vitamin D as an Adjunctive Treatment to Standard Drugs in Pulmonary Tuberculosis Patients: An Evidence-Based Case Report. Adv. Prev. Med. 2019, 2019, 5181847. [Google Scholar] [CrossRef] [PubMed]
- Schutz, C.; Davis, A.G.; Sossen, B.; Lai, R.P.J.; Ntsekhe, M.; Harley, Y.X.R.; Wilkinson, R.J. Corticosteroids as an adjunct to tuberculosis therapy. Expert Rev. Respir. Med. 2018, 12, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Kumarvelu, S.; Prasad, K.; Khosla, A.; Behari, M.; Ahuja, G.K. Randomized controlled trial of dexamethasone in tuberculous meningitis. Tuber. Lung Dis. 1994, 75, 203–207. [Google Scholar] [CrossRef]
- Bekele, A.; Gebreselassie, N.; Ashenafi, S.; Kassa, E.; Amogne, W.; Tefera, M.; Aseffa, A.; Worku, A.; Raqib, R.; Agerberth, B.; et al. Daily adjunctive therapy with vitamin D3 and phenylbutyrate supports clinical recovery from pulmonary tuberculosis: A randomized controlled trial in Ethiopia. J. Intern Med. 2019, 284, 292–306. [Google Scholar] [CrossRef]
- Rao, M.; Valentini, D.; Zumla, A.; Maeurer, M. Evaluation of the efficacy of valproic acid and suberoylanilide hydroxamic acid (vorinostat) in enhancing the effects of first-line tuberculosis drugs against intracellular Mycobacterium tuberculosis. Int. J. Infect. Dis. 2018, 69, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Mily, A.; Rekha, R.S.; Kamal, S.M.M.; Arifuzzaman, A.S.M.; Rahim, Z.; Khan, L.; Haq, M.A.; Zaman, K.; Bergman, P.; Brighenti, S.; et al. Significant effects of oral phenylbutyrate and Vitamin D3 adjunctive therapy in pulmonary tuberculosis: A randomized controlled trial. PLoS ONE 2015, 10, e0138340. [Google Scholar] [CrossRef]
- Congreve, M.; Chessari, G.; Tisi, D.; Woodhead, A.J. Recent Developments in Fragment-Based Drug Discovery. J. Med. Chem. 2008, 51, 3661–3680. [Google Scholar] [CrossRef]
- Schoeman, J.F.; Springer, P.; van Rensburg, A.J.; Swanevelder, S.; Hanekom, W.A.; Haslett, P.A.J.; Kaplan, G. Adjunctive thalidomide therapy for childhood tuberculous meningitis: Results of a randomized study. J. Child Neurol. 2004, 19, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kolloli, A.; Singh, P.; Vinnard, C.; Kaplan, G.; Subbian, S. Thalidomide and Phosphodiesterase 4 Inhibitors as Host Directed Therapeutics for Tuberculous Meningitis: Insights From the Rabbit Model. Front. Cell. Infect. Microbiol. 2020, 9, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabir, N.; Hussain, T.; Mangi, M.H.; Zhao, D.; Zhou, X. Matrix metalloproteinases: Expression, regulation and role in the immunopathology of tuberculosis. Cell Prolif. 2019, 52, e12649. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, L.; Zimmerman, M.D.; Chen, K.-Y.; Huang, L.; Fu, D.-J.; Kaya, F.; Rakhilin, N.; Nazarova, E.V.; Bu, P.; et al. Matrix metalloproteinase inhibitors enhance the efficacy of frontline drugs against Mycobacterium tuberculosis. PLoS Pathog. 2018, 14, e1006974. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Brumell, J.H. Bacteria–autophagy interplay: A battle for survival. Nat. Rev. Microbiol. 2014, 12, 101–114. [Google Scholar] [CrossRef]
- Chen, C.; Gardete, S.; Jansen, R.S.; Shetty, A.; Dick, T.; Rhee, K.Y.; Dartoisa, V. Verapamil targets membrane energetics in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e02107-17. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Tasneen, R.; Peloquin, C.A.; Almeida, D.V.; Li, S.Y.; Barnes-Boyle, K.; Lu, Y.; Nuermberger, E. Verapamil increases the bioavailability and efficacy of bedaquiline but not clofazimine in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2018, 62, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dutta, N.K.; Bruiners, N.; Zimmerman, M.D.; Tan, S.; Dartois, V.; Gennaro, M.L.; Karakousis, P.C. Adjunctive host-directed therapy with statins improves tuberculosis-related outcomes in mice. J. Infect. Dis. 2020, 221, 1079–1087. [Google Scholar] [CrossRef]
- Magee, M.J.; Salindri, A.D.; Kornfeld, H.; Singhal, A. Reduced prevalence of latent tuberculosis infection in diabetes patients using metformin and statins. Eur. Respir. J. 2019, 53, 1–4. [Google Scholar] [CrossRef]
- Hoffmann, P.; Roumeguère, T.; Schulman, C.; Van Velthoven, R. Use of statins and outcome of BCG treatment for bladder cancer. N. Engl. J. Med. 2006, 355, 2705–2707. [Google Scholar] [CrossRef]
- Cilloniz, C.; Torres, A. Statins as Adjunctive Therapy Against Tuberculosis (TB): The Balance Between Statin-Induced Anti-TB Effect and Trained Immunity Suppression To. J. Infect. Dis. 2020, 222, 334–335. [Google Scholar] [CrossRef]
- Naicker, N.; Sigal, A.; Naidoo, K. Metformin as Host-Directed Therapy for TB Treatment: Scoping Review. Front. Microbiol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Singhal, A.; Jie, L.; Kumar, P.; Hong, G.S.; Leow, M.K.S.; Paleja, B.; Tsenova, L.; Kurepina, N.; Chen, J.; Zolezzi, F.; et al. Metformin as adjunct antituberculosis therapy. Sci. Transl. Med. 2014, 6, 263ra159. [Google Scholar] [CrossRef]
- Lee, Y.J.; Han, S.K.; Park, J.H.; Lee, J.K.; Kim, D.K.; Chung, H.S.; Heo, E.Y. The effect of metformin on culture conversion in tuberculosis patients with diabetes mellitus. Korean J. Intern. Med. 2018, 33, 933–940. [Google Scholar] [CrossRef]
- Degner, N.R.; Wang, J.Y.; Golub, J.E.; Karakousis, P.C. Metformin Use Reverses the Increased Mortality Associated with Diabetes Mellitus during Tuberculosis Treatment. Clin. Infect. Dis. 2018, 66, 198–205. [Google Scholar] [CrossRef]
- Maiga, M.C.; Ahidjo, B.A.; Maiga, M.; Bishai, W.R. Roflumilast, a Type 4 phosphodiesterase inhibitor, shows promising adjunctive, host-directed therapeutic activity in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 7888–7890. [Google Scholar] [CrossRef] [Green Version]
- Maiga, M.; Agarwal, N.; Ammerman, N.C.; Gupta, R.; Guo, H.; Maiga, M.C.; Lun, S.; Bishai, W.R. Successful shortening of tuberculosis treatment using adjuvant host-directed therapy with FDA-approved phosphodiesterase inhibitors in the mouse model. PLoS ONE 2012, 7, e30749. [Google Scholar] [CrossRef] [Green Version]
- Leukes, V.; Walzl, G.; du Plessis, N. Myeloid-Derived Suppressor Cells as Target of Phosphodiesterase-5 Inhibitors in Host-Directed Therapeutics for Tuberculosis. Front. Immunol. 2020, 11, 1–7. [Google Scholar] [CrossRef]
- Subbian, S.; Tsenova, L.; Holloway, J.; Peixoto, B.; O’Brien, P.; Dartois, V.; Khetani, V.; Zeldis, J.B.; Kaplan, G. Adjunctive Phosphodiesterase-4 Inhibitor Therapy Improves Antibiotic Response to Pulmonary Tuberculosis in a Rabbit Model. EBioMedicine 2016, 4, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Subbian, S.; Tsenova, L.; O’Brien, P.; Yang, G.; Koo, M.S.; Peixoto, B.; Fallows, D.; Zeldis, J.B.; Muller, G.; Kaplan, G. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am. J. Pathol. 2011, 179, 289–301. [Google Scholar] [CrossRef]
- Napier, R.J.; Rafi, W.; Cheruvu, M.; Powell, K.R.; Zaunbrecher, M.A.; Bornmann, W.; Salgame, P.; Shinnick, T.M.; Kalman, D. Imatinib-Sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis. Cell Host Microbe 2011, 10, 475–485. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Rao, N.; Malhotra, K.P. Renal tuberculosis in an imatinib-treated chronic myeloid leukemia. Braz. J. Nephrol. 2020, 42, 366–369. [Google Scholar] [CrossRef]
- Arbués, A.; Brees, D.; Chibout, S.D.; Fox, T.; Kammüller, M.; Portevin, D. TNF-α antagonists differentially induce TGFβ1-dependent resuscitation of dormant-like Mycobacterium tuberculosis. PLoS Pathog. 2020, 16, e1008312. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Zhao, D.; Shah, S.Z.A.; Sabir, N.; Wang, J.; Liao, Y.; Song, Y.; Dong, H.; Hussain Mangi, M.; Ni, J.; et al. Nilotinib: A Tyrosine Kinase Inhibitor Mediates Resistance to Intracellular Mycobacterium Via Regulating Autophagy. Cells 2019, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Sogi, K.M.; Lien, K.A.; Johnson, J.R.; Krogan, N.J.; Stanley, S.A. The Tyrosine Kinase Inhibitor Gefitinib Restricts Mycobacterium tuberculosis Growth through Increased Lysosomal Biogenesis and Modulation of Cytokine Signaling. ACS Infect. Dis. 2017, 3, 564–574. [Google Scholar] [CrossRef]
- Mourenza, Á.; Gil, J.A.; Mateos, L.M.; Letek, M. Novel Treatments against Mycobacterium tuberculosis Based on Drug Repurposing. Antibiotics 2020, 9, 550. [Google Scholar] [CrossRef]
- Gupta, S.; Cheung, L.; Pokkali, S.; Winglee, K.; Guo, H.; Murphy, J.R.; Bishai, W.R. Suppressor Cell-Depleting Immunotherapy with Denileukin Diftitox is an Effective Host-Directed Therapy for Tuberculosis. J. Infect. Dis. 2017, 215, 1883–1887. [Google Scholar] [CrossRef] [Green Version]
- Dobler, C.C. Biologic Agents and Tuberculosis. Microbiol. Spectr. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Parida, S.K.; Poiret, T.; Zhenjiang, L.; Meng, Q.; Heyckendorf, J.; Lange, C.; Ambati, A.S.; Rao, M.V.; Valentini, D.; Ferrara, G.; et al. T-Cell Therapy: Options for Infectious Diseases. Clin. Infect. Dis. 2015, 61, S217–S224. [Google Scholar] [CrossRef] [Green Version]
- Pushkaran, A.C.; Vinod, V.; Vanuopadath, M.; Nair, S.S.; Nair, S.V.; Vasudevan, A.K.; Biswas, R.; Mohan, C.G. Combination of Repurposed Drug Diosmin with Amoxicillin-Clavulanic acid Causes Synergistic Inhibition of Mycobacterial Growth. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Barber, K.D.; Andrade, B.B.; Oland, S.D.; Amaral, E.P.; Barber, D.L.; Gonzales, J.; Derrick, S.C.; Shi, R.; Pavan, N. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2016, 511, 99–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.K.; Yabaji, S.M.; Dubey, R.K. Evaluation of isoprinosine to be repurposed as an adjunct anti-tuberculosis chemotherapy. Med. Hypotheses 2018, 115, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Purhonen, M.; Koponen, H.; Tiihonen, J.; Tanskanen, A. Determinants of cholesterol and triglycerides recording in patients treated with lipid lowering therapy in UK primary care. Pharmacoepidemiol. Drug Saf. 2007, 16, 228. [Google Scholar]
- Amaral, L.; Viveiros, M. Why thioridazine in combination with antibiotics cures extensively drug-resistant Mycobacterium tuberculosis infections. Int. J. Antimicrob. Agents 2012, 39, 376–380. [Google Scholar] [CrossRef]
- Dalecki, A.G.; Haeili, M.; Shah, S.; Speer, A.; Niederweis, M.; Kutsch, O.; Wolschendorf, F. Disulfiram and copper ions kill Mycobacterium tuberculosis in a synergistic manner. Antimicrob. Agents Chemother. 2015, 59, 4835–4844. [Google Scholar] [CrossRef] [Green Version]
- Ivanyi, J.; Zumla, A. Nonsteroidal antiinflammatory drugs for adjunctive tuberculosis treatment. J. Infect. Dis. 2013, 208, 185–188. [Google Scholar] [CrossRef]
- Guzman, J.D.; Evangelopoulos, D.; Gupta, A.; Birchall, K.; Mwaigwisya, S.; Saxty, B.; McHugh, T.D.; Gibbons, S.; Malkinson, J.; Bhakta, S. Antitubercular specific activity of ibuprofen and the other 2-arylpropanoic acids using the HT-SPOTi whole-cell phenotypic assay. BMJ Open 2013, 3, e002672. [Google Scholar] [CrossRef] [Green Version]
- Kroesen, V.M.; Rodríguez-Martínez, P.; García, E.; Rosales, Y.; Díaz, J.; Martín-Céspedes, M.; Tapia, G.; Sarrias, M.R.; Cardona, P.J.; Vilaplana, C. A beneficial effect of low-dose aspirin in a murine model of active tuberculosis. Front. Immunol. 2018, 9, 798. [Google Scholar] [CrossRef] [Green Version]
- Vilaplana, C.; Marzo, E.; Tapia, G.; Diaz, J.; Garcia, V.; Cardona, P.J. Ibuprofen therapy resulted in significantly decreased tissue bacillary loads and increased survival in a new murine experimental model of active tuberculosis. J. Infect. Dis. 2013, 208, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, R.; Clemmensen, H.S.; Woodworth, J.S.; Therkelsen, M.L.; Mustafa, T.; Tonby, K.; Jenum, S.; Agger, E.M.; Dyrhol-Riise, A.M.; Andersen, P. Cyclooxygenase inhibitors impair CD4 T cell immunity and exacerbate Mycobacterium tuberculosis infection in aerosol-challenged mice. Commun. Biol. 2019, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Byrne, S.T.; Denkin, S.M.; Zhang, Y. Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J. Antimicrob. Chemother. 2007, 59, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.T.; Denkin, S.M.; Zhang, Y. Aspirin antagonism in isoniazid treatment of tuberculosis in mice. Antimicrob. Agents Chemother. 2007, 51, 794–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, U.; Kalita, J.; Sagar, B.; Bhoi, S. Does adjunctive corticosteroid and aspirin therapy improve the outcome of tuberculous meningitis? Neurol. India 2018, 66, 1672–1677. [Google Scholar] [PubMed]
- Gold, B.; Pingle, M.; Brickner, S.J.; Shah, N.; Roberts, J.; Rundell, M.; Bracken, W.C.; Warrier, T.; Somersan, S.; Venugopal, A.; et al. Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc. Natl. Acad. Sci. USA 2012, 109, 16004–16011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldkind, L.; Laine, L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: Lessons learned from the bromfenac experience. Pharmacoepidemiol. Drug Saf. 2006, 15, 213–220. [Google Scholar] [CrossRef]
- Dutta, N.K.; Mazumdar, K.; Dastidar, S.G.; Park, J.H. Activity of diclofenac used alone and in combination with streptomycin against Mycobacterium tuberculosis in mice. Int. J. Antimicrob. Agents 2007, 30, 336–340. [Google Scholar] [CrossRef]
- Maitra, A.; Evangelopoulos, D.; Chrzastek, A.; Martin, L.T.; Hanrath, A.; Chapman, E.; Hailes, H.C.; Lipman, M.; McHugh, T.D.; Waddell, S.J.; et al. Carprofen elicits pleiotropic mechanisms of bactericidal action with the potential to reverse antimicrobial drug resistance in tuberculosis. J. Antimicrob. Chemother. 2020, 75, 3194–3201. [Google Scholar] [CrossRef]
- The European Agency for the Evaluation of Medicinal Products: Carprofen Summary Report. Available online: https://www.ema.europa.eu/en/documents/mrl-report/carprofen-summary-report-1-committee-veterinary-medicinal-products_en.pdf (accessed on 16 December 2020).
- Kana, B.D.; Karakousis, P.C.; Parish, T.; Dick, T. Future target-based drug discovery for tuberculosis? Tuberculosis 2014, 94, 551–556. [Google Scholar] [CrossRef]
- Ryan, A.; Polycarpou, E.; Lack, N.A.; Evangelopoulos, D.; Sieg, C.; Halman, A.; Bhakta, S.; Eleftheriadou, O.; McHugh, T.D.; Keany, S.; et al. Investigation of the mycobacterial enzyme HsaD as a potential novel target for anti-tubercular agents using a fragment-based drug design approach. Br. J. Pharmacol. 2017, 174, 2209–2224. [Google Scholar] [CrossRef]
- Kumar, S.; Bansal, K.; Holla, S.; Verma-Kumar, S.; Sharma, P.; Balaji, K.N. ESAT-6 induced COX-2 expression involves coordinated interplay between PI3K and MAPK signaling. Mol. Immunol. 2012, 49, 655–663. [Google Scholar]
- Harbut, M.B.; Vilchèze, C.; Luo, X.; Hensler, M.E.; Guo, H.; Yang, B.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Bhakta, S. The Prospect of Repurposing Immunomodulatory Drugs for Adjunctive Chemotherapy against Tuberculosis: A Critical Review. Antibiotics 2021, 10, 91. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010091
Lee C, Bhakta S. The Prospect of Repurposing Immunomodulatory Drugs for Adjunctive Chemotherapy against Tuberculosis: A Critical Review. Antibiotics. 2021; 10(1):91. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010091
Chicago/Turabian StyleLee, Chiyun, and Sanjib Bhakta. 2021. "The Prospect of Repurposing Immunomodulatory Drugs for Adjunctive Chemotherapy against Tuberculosis: A Critical Review" Antibiotics 10, no. 1: 91. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10010091






