Anti-Virulence Therapeutic Approaches for Neisseria gonorrhoeae
Abstract
:1. Introduction
2. Treatment and Antimicrobial Resistance
3. Pathogenesis Mechanisms of N. gonorrhoeae
4. Resistance of Gonococcus to Killing by Macrophages and PMNs
5. AVTs as an Intervention Strategy
6. Gonococcal Virulence Factors as Targets for Inhibitor Design
6.1. Bacterial Cell Wall Maintenance and Modification
6.1.1. Lipid A Phosphoethanolamine Transferase
6.1.2. LOS Sialyltransferase
6.1.3. Lysozyme Inhibitors
6.1.4. PG O-Acetyltransferase B
6.1.5. Lytic Transglycosylase A
6.2. Anaerobic Survival
6.3. Efflux Pumps
6.4. Protein Folding Pathways
6.4.1. Macrophage Infectivity Potentiator
6.4.2. Oxidative Protein Folding System
6.5. Adhesion and Invasion
6.5.1. Type IV Pili
6.5.2. Mannose-Binding (Opa) Proteins
7. Considerations for Further Clinical Development of AVTs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018; Department of Health and Human Services: Atlanta, GA, USA, 2019. [CrossRef]
- Owusu-Edusei, K.J.; Chesson, H.W.; Gift, T.L.; Tao, G.; Mahajan, R.; Ocfemia, M.C.B.; Kent, C.K. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex. Transm. Dis. 2013, 40, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Sherrard, J.; Barlow, D. Gonorrhoea in men: Clinical and diagnostic aspects. Genitourin. Med. 1996, 72, 422–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kent, C.K.; Chaw, J.K.; Wong, W.; Liska, S.; Gibson, S.; Hubbard, G.; Klausner, J.D. Prevalence of rectal, urethral, and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin. Infect. Dis. 2005, 41, 67–74. [Google Scholar] [CrossRef]
- Morris, S.R.; Klausner, J.D.; Buchbinder, S.P.; Wheeler, S.L.; Koblin, B.; Coates, T.; Chesney, M.; Colfax, G.N. Prevalence and incidence of pharyngeal gonorrhea in a longitudinal sample of men who have sex with men: The EXPLORE study. Clin. Infect. Dis. 2006, 43, 1284–1289. [Google Scholar] [CrossRef] [Green Version]
- Kinghorn, G. Pharyngeal gonorrhoea: A silent cause for concern. Sex. Transm. Infect. 2010, 86, 413–414. [Google Scholar] [CrossRef]
- Peters, R.P.; Verweij, S.P.; Nijsten, N.; Ouburg, S.; Mutsaers, J.; Jansen, C.L.; van Leeuwen, A.P.; Morré, S.A. Evaluation of sexual history-based screening of anatomic sites for Chlamydia trachomatis and Neisseria gonorrhoeae infection in men having sex with men in routine practice. BMC Infect. Dis. 2011, 11, 203. [Google Scholar] [CrossRef] [Green Version]
- McCormack, W.M.; Johnson, K.; Stumacher, R.J.; Donner, A.; Rychwalski, R. Clinical spectrum of gonococcal infection in women. Lancet 1977, 309, 1182–1185. [Google Scholar] [CrossRef]
- Walker, C.K.; Sweet, R.L. Gonorrhea infection in women: Prevalence, effects, screening, and management. Int. J. Womens Health 2011, 3, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Rapista, A.; Teleshova, N.; Mosoyan, G.; Jarvis, G.A.; Klotman, M.E.; Chang, T.L. Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation. J. Immunol. 2010, 184, 2814–2824. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, G.A.; Chang, T.L. Modulation of HIV transmission by Neisseria gonorrhoeae: Molecular and immunological aspects. Curr. HIV Res. 2012, 10, 211–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malott, R.J.; Keller, B.O.; Gaudet, R.G.; McCaw, S.E.; Lai, C.C.; Dobson-Belaire, W.N.; Hobbs, J.L.; Michael, F.S.; Cox, A.D.; Moraes, T.F. Neisseria gonorrhoeae-derived heptose elicits an innate immune response and drives HIV-1 expression. Proc. Natl. Acad. Sci. USA 2013, 110, 10234–10239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanyal, A.; Shen, C.; Ding, M.; Reinhart, T.A.; Chen, Y.; Sankapal, S.; Gupta, P. Neisseria gonorrhoeae uses cellular proteins CXCL10 and IL8 to enhance HIV-1 transmission across cervical mucosa. Am. J. Reprod. Immunol. 2019, 81, e13111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guvenc, F.; Kaul, R.; Gray-Owen, S.D. Intimate relations: Molecular and immunologic interactions between Neisseria gonorrhoeae and HIV-1. Front. Microbiol. 2020, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, G.L.; Watson, W.J. Preterm premature rupture of membranes: Results of expectant management in patients with cervical cultures positive for group B streptococcus or Neisseria gonorrhoeae. Am. J. Obstet. Gynecol. 1992, 166, 945–949. [Google Scholar] [CrossRef]
- Heumann, C.L.; Quilter, L.A.S.; Eastment, M.C.; Heffron, R.; Hawes, S.E. Adverse birth outcomes and maternal Neisseria gonorrhoeae infection: A population-based cohort study in Washington State. Sex. Transm. Dis. 2017, 44, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Thompson, T.R.; Swanson, R.E.; Wiesner, P.J. Gonococcal ophthalmia neonatorum: Relationship of time of infection to relevant control measures. JAMA 1974, 228, 186–188. [Google Scholar] [CrossRef]
- Rees, E.; Tait, I.A.; Hobson, D.; Byng, R.E.; Johnson, F.W. Neonatal conjunctivitis caused by Neisseria gonorrhoeae and Chlamydia trachomatis. Sex. Transm. Infect. 1977, 53, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Laga, M.; Meheus, A.; Piot, P. Epidemiology and control of gonococcal ophthalmia neonatorum. Bull. World Health Organ. 1989, 67, 471–477. [Google Scholar]
- Epling, J. Bacterial conjunctivitis. BMJ Clin. Evid. 2012, 2012, 0704. [Google Scholar]
- Fung, M.; Scott, K.C.; Kent, C.K.; Klausner, J.D. Chlamydial and gonococcal reinfection among men: A systematic review of data to evaluate the need for retesting. Sex. Transm. Infect. 2007, 83, 304–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerse, A.E.; Bash, M.C.; Russell, M.W. Vaccines against gonorrhea: Current status and future challenges. Vaccine 2014, 32, 1579–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petousis-Harris, H.; Paynter, J.; Morgan, J.; Saxton, P.; McArdle, B.; Goodyear-Smith, F.; Black, S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet 2017, 390, 1603–1610. [Google Scholar] [CrossRef]
- Semchenko, E.A.; Tan, A.; Borrow, R.; Seib, K.L. The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae. Clin. Infect. Dis. 2019, 69, 1101–1111. [Google Scholar] [CrossRef]
- Gottlieb, S.L.; Jerse, A.E.; Delany-Moretlwe, S.; Deal, C.; Giersing, B.K. Advancing vaccine development for gonorrhoea and the Global STI Vaccine Roadmap. Sex. Health 2019, 16, 426–432. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for the Treatment of Neisseria gonorrhoeae; World Health Organization: Geneva, Switzerland, 2016; Available online: http://www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/ (accessed on 18 September 2020).
- Workowski, K.A.; Bolan, G.A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2015, 64, 1–137. [Google Scholar]
- Ison, C.A. Biology of Neisseria gonorrhoeae and the clinical picture of infection. In Sexually Transmitted Infections and Sexually Transmitted Diseases; Gross, G.E., Tyring, S.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–90. ISBN 978-3-642-14663-3. [Google Scholar]
- Centers for Disease Control and Prevention. Cephalosporin susceptibility among Neisseria gonorrhoeae isolates—United States, 2000–2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 873–877. [Google Scholar]
- Unemo, M.; Nicholas, R.A. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol. 2012, 7, 1401–1422. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. CDC grand rounds: The growing threat of multidrug-resistant gonorrhea. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 103–106. [Google Scholar]
- Unemo, M.; Golparian, D.; Hellmark, B. First three Neisseria gonorrhoeae isolates with high-level resistance to azithromycin in Sweden: A threat to currently available dual-antimicrobial regimens for treatment of gonorrhea? Antimicrob. Agents Chemother. 2014, 58, 624–625. [Google Scholar] [CrossRef] [Green Version]
- Fifer, H.; Natarajan, U.; Jones, L.; Alexander, S.; Hughes, G.; Golparian, D.; Unemo, M. Failure of dual antimicrobial therapy in treatment of gonorrhea. N. Engl. J. Med. 2016, 374, 2504–2506. [Google Scholar] [CrossRef] [PubMed]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.-A.R.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, A.R.; Komeya, A.Y.; Kirkcaldy, R.D.; Whelen, A.C.; Soge, O.O.; Papp, J.R.; Kersh, E.N.; Wasserman, G.M.; O’Connor, N.P.; O’Brien, P.S.; et al. Cluster of Neisseria gonorrhoeae isolates with high-level azithromycin resistance and decreased ceftriaxone susceptibility, Hawaii, 2016. Clin. Infect. Dis. 2017, 65, 918–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahra, M.M.; Martin, I.; Demczuk, W.; Jennison, A.V.; Lee, K.-I.; Nakayama, S.-I.; Lefebvre, B.; Longtin, J.; Ward, A.; Mulvey, M.R.; et al. Cooperative recognition of internationally disseminated ceftriaxone-resistant Neisseria gonorrhoeae strain. Emerg. Infect. Dis. 2018, 24, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Eyre, D.W.; Sanderson, N.D.; Lord, E.; Regisford-Reimmer, N.; Chau, K.; Barker, L.; Morgan, M.; Newnham, R.; Golparian, D.; Unemo, M.; et al. Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England, February 2018. Eurosurveillance 2018, 23, 1800323. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019; Department of Health and Human Services: Atlanta, GA, USA, 2019. [CrossRef] [Green Version]
- Ko, K.K.K.; Chio, M.T.W.; Goh, S.S.; Tan, A.L.; Koh, T.H.; Rahman, N.B.A. First case of ceftriaxone-resistant multidrug-resistant Neisseria gonorrhoeae in Singapore. Antimicrob. Agents Chemother. 2019, 63, e02624-18. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 2006, 42, S82–S89. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.R.; Hota, B.; Ahmad, I.; Scott, R.D.; Foster, S.D.; Abbasi, F.; Schabowski, S.; Kampe, L.M.; Ciavarella, G.G.; Supino, M.; et al. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: Implications for antibiotic stewardship. Clin. Infect. Dis. 2009, 49, 1175–1184. [Google Scholar] [CrossRef]
- Mainous, A.G.; Diaz, V.A.; Matheson, E.M.; Gregorie, S.H.; Hueston, W.J. Trends in hospitalizations with antibiotic-resistant infections: U.S., 1997–2006. Public Health Rep. 2011, 126, 354–360. [Google Scholar] [CrossRef]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Opatowski, M.; Tuppin, P.; Cosker, K.; Touat, M.; Lagasnerie, G.D.; Guillemot, D.; Salomon, J.; Brun-Buisson, C.; Watier, L. Hospitalisations with infections related to antimicrobial-resistant bacteria from the French nationwide hospital discharge database, 2016. Epidemiol. Infect. 2019, 147, e144. [Google Scholar] [CrossRef] [Green Version]
- Golparian, D.; Shafer, W.M.; Ohnishi, M.; Unemo, M. Importance of multidrug efflux pumps in the antimicrobial resistance property of clinical multidrug-resistant isolates of Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 2014, 58, 3556–3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.N.; Morris, D.H.; Avery, A.K.; Workowski, K.A.; Batteiger, B.E.; Tiffany, C.A.; Perry, C.R.; Raychaudhuri, A.; Scangarella-Oman, N.E.; Hossain, M.; et al. Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: A phase 2, randomized, dose-ranging, single-oral dose evaluation. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 67, 504–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.N.; Marrazzo, J.; Batteiger, B.E.; Hook, E.W.; Seña, A.C.; Long, J.; Wierzbicki, M.R.; Kwak, H.; Johnson, S.M.; Lawrence, K.; et al. Single-dose zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N. Engl. J. Med. 2018, 379, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; McNulty, A.; Avery, A.; Whiley, D.; Tabrizi, S.N.; Hardy, D.; Das, A.F.; Nenninger, A.; Fairley, C.K.; Hocking, J.S.; et al. Solithromycin versus ceftriaxone plus azithromycin for the treatment of uncomplicated genital gonorrhoea (SOLITAIRE-U): A randomised phase 3 non-inferiority trial. Lancet Infect. Dis. 2019, 19, 833–842. [Google Scholar] [CrossRef]
- Jacobsson, S.; Mason, C.; Khan, N.; Meo, P.; Unemo, M. In vitro activity of the novel oral antimicrobial SMT-571, with a new mechanism of action, against MDR and XDR Neisseria gonorrhoeae: Future treatment option for gonorrhoea? J. Antimicrob. Chemother. 2019, 74, 1591–1594. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.S. Treating uncomplicated Neisseria gonorrhoeae infections: Is the anatomic site of infection important? Sex. Transm. Dis. 1995, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Manavi, K.; Young, H.; McMillan, A. The outcome of oropharyngeal gonorrhoea treatment with different regimens. Int. J. STD AIDS 2005, 16, 68–70. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Walker, S.; Hocking, J.S.; Bradshaw, C.S.; Chen, M.Y.; Tabrizi, S.N.; Howden, B.P.; Law, M.G.; Maddaford, K.; Read, T.R.H.; et al. A multicentre double-blind randomised controlled trial evaluating the efficacy of daily use of antibacterial mouthwash against oropharyngeal gonorrhoea among men who have sex with men: The OMEGA (Oral Mouthwash use to Eradicate GonorrhoeA) study protocol. BMC Infect. Dis. 2017, 17, 456. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Williamson, D.A.; Hocking, J.S.; Law, M.G.; Maddaford, K.; Bradshaw, C.S.; McNulty, A.; Templeton, D.J.; Moore, R.; Murray, G.L.; et al. Antiseptic mouthwash for gonorrhoea prevention (OMEGA): A randomised, double-blind, parallel-group, multicentre trial. Sex. Health 2020, 17, viii. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Maddaford, K.; Hocking, J.S.; Bradshaw, C.S.; Wigan, R.; Chen, M.Y.; Howden, B.P.; Williamson, D.A.; Fairley, C.K. An open-label, parallel-group, randomised controlled trial of antiseptic mouthwash versus antibiotics for oropharyngeal gonorrhoea treatment (OMEGA2). Sci. Rep. 2020, 10, 19386. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-C.; Christodoulides, M. The biology of Neisseria adhesins. Biology 2013, 2, 1054–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifert, H.S. Questions about gonococcal pilus phase- and antigenic variation. Mol. Microbiol. 1996, 21, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.A.; Masters, T.L.; Wachter, J. Gonorrhea—An evolving disease of the new millennium. Microb. Cell 2016, 3, 371. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, M.; Heuer, D.; Meyer, T.F. CD46-independent binding of neisserial type IV pili and the major pilus adhesin, PilC, to human epithelial cells. Infect. Immun. 2005, 73, 3072. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, A.-B.; Ilver, D.; Falk, P.; Pepose, J.; Normark, S. Sequence changes in the pilus subunit lead to tropism variation of Neisseria gonorrhoeae to human tissue. Mol. Microbiol. 1994, 13, 403–416. [Google Scholar] [CrossRef]
- Long, C.D.; Madraswala, R.N.; Seifert, H.S. Comparisons between colony phase variation of Neisseria gonorrhoeae FA1090 and pilus, pilin, and S-pilin expression. Infect. Immun. 1998, 66, 1918–1927. [Google Scholar] [CrossRef] [Green Version]
- Stern, A.; Brown, M.; Nickel, P.; Meyer, T.F. Opacity genes in Neisseria gonorrhoeae: Control of phase and antigenic variation. Cell 1986, 47, 61–71. [Google Scholar] [CrossRef]
- Edwards, J.L.; Apicella, M.A. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 2004, 17, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Quillin, S.J.; Seifert, H.S. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat. Rev. Microbiol. 2018, 16, 226–240. [Google Scholar] [CrossRef]
- Van Putten, J.P.; Paul, S.M. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeae entry into human mucosal cells. EMBO J. 1995, 14, 2144–2154. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Belland, R.J.; Wilson, J.; Swanson, J. Adherence of pilus-Opa+ gonococci to epithelial cells in vitro involves heparan sulfate. J. Exp. Med. 1995, 182, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porat, N.; Apicella, M.A.; Blake, M.S. Neisseria gonorrhoeae utilizes and enhances the biosynthesis of the asialoglycoprotein receptor expressed on the surface of the hepatic HepG2 cell line. Infect. Immun. 1995, 63, 1498–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, H.A.; Porat, N.; Campbell, C.A.; Jennings, M.; Gibson, B.W.; Phillips, N.J.; Apicella, M.A.; Blake, M.S. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol. Microbiol. 2000, 36, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Harvey, H.A.; Jennings, M.P.; Campbell, C.A.; Williams, R.; Apicella, M.A. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: The role of the asialoglycoprotein receptor. Mol. Microbiol. 2001, 42, 659–672. [Google Scholar] [CrossRef]
- Higashi, D.L.; Lee, S.W.; Snyder, A.; Weyand, N.J.; Bakke, A.; So, M. Dynamics of Neisseria gonorrhoeae attachment: Microcolony development, cortical plaque formation, and cytoprotection. Infect. Immun. 2007, 75, 4743–4753. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.T.; Byerly, L.; Apicella, M.A.; Seifert, H.S. Seminal plasma promotes Neisseria gonorrhoeae aggregation and biofilm formation. J. Bacteriol. 2016, 198, 2228–2235. [Google Scholar] [CrossRef] [Green Version]
- Merz, A.J.; Rifenbery, D.B.; Arvidson, C.G.; So, M. Traversal of a polarized epithelium by pathogenic Neisseriae: Facilitation by type IV pili and maintenance of epithelial barrier function. Mol. Med. 1996, 2, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gray-Owen, S.D.; Knorre, A.; Meyer, T.F.; Dehio, C. Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol. Microbiol. 1998, 30, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Ilver, D.; Källström, H.; Normark, S.; Jonsson, A.-B. Transcellular passage of Neisseria gonorrhoeae involves pilus phase variation. Infect. Immun. 1998, 66, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.K.; Rosenthal, R.S. Release of soluble peptidoglycan from growing gonococci: Demonstration of anhydro-muramyl-containing fragments. Infect. Immun. 1980, 29, 914–925. [Google Scholar] [PubMed]
- Kaparakis, M.; Turnbull, L.; Carneiro, L.; Firth, S.; Coleman, H.A.; Parkington, H.C.; Bourhis, L.L.; Karrar, A.; Viala, J.; Mak, J.; et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cell. Microbiol. 2010, 12, 372–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; John, C.M.; Jarvis, G.A. Phosphoryl moieties of lipid A from Neisseria meningitidis and N. gonorrhoeae lipooligosaccharides play an important role in activation of both MyD88- and TRIF-Dependent TLR4–MD-2 signaling pathways. J. Immunol. 2010, 185, 6974–6984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrogiorgos, N.; Mekasha, S.; Yang, Y.; Kelliher, M.A.; Ingalls, R.R. Activation of NOD receptors by Neisseria gonorrhoeae modulates the innate immune response. Innate Immun. 2014, 20, 377–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudet, R.G.; Sintsova, A.; Buckwalter, C.M.; Leung, N.; Cochrane, A.; Li, J.; Cox, A.D.; Moffat, J.; Gray-Owen, S.D. Cytosolic detection of the bacterial metabolite HBP activates TIFA-dependent innate immunity. Science 2015, 348, 1251–1255. [Google Scholar] [CrossRef]
- Pachathundikandi, K.; Backert, S. Heptose 1,7-bisphosphate directed TIFA oligomerization: A novel PAMP-recognizing signaling platform in the control of bacterial infections. Gastroenterology 2018, 154, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, K.H.; Schneider, H.; Cross, A.S.; Boslego, J.W.; Hoover, D.L.; Staley, T.L.; Kuschner, R.A.; Deal, C.D. Inflammatory cytokines produced in response to experimental human gonorrhea. J. Infect. Dis. 1995, 172, 186–191. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Kandler, J.L.; Balthazar, J.T.; Shafer, W.M. Phosphoethanolamine modification of Neisseria gonorrhoeae lipid A reduces autophagy flux in macrophages. PLoS ONE 2015, 10, e0144347. [Google Scholar] [CrossRef]
- Criss, A.K.; Seifert, H.S. A bacterial siren song: Intimate interactions between Neisseria and neutrophils. Nat. Rev. Microbiol. 2012, 10, 178–190. [Google Scholar] [CrossRef]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Levy, O. Antimicrobial proteins and peptides: Anti-infective molecules of mammalian leukocytes. J. Leukoc. Biol. 2004, 76, 909–925. [Google Scholar] [CrossRef]
- Tseng, H.-J.; Srikhanta, Y.; McEwan, A.G.; Jennings, M.P. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 2001, 40, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Claverys, J.-P. A new family of high-affinity ABC manganese and zinc permeases. Res. Microbiol. 2001, 152, 231–243. [Google Scholar] [CrossRef]
- Seib, K.L.; Wu, H.-J.; Kidd, S.P.; Apicella, M.A.; Jennings, M.P.; McEwan, A.G. Defenses against oxidative stress in Neisseria gonorrhoeae: A system tailored for a challenging environment. Microbiol. Mol. Biol. Rev. 2006, 70, 344–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seib, K.L.; Tseng, H.-J.; McEwan, A.G.; Apicella, M.A.; Jennings, M.P. Defenses against oxidative stress in Neisseria gonorrhoeae and Neisseria meningitidis: Distinctive systems for different lifestyles. J. Infect. Dis. 2004, 190, 136–147. [Google Scholar] [CrossRef] [Green Version]
- Archibald, F.S.; Duong, M.N. Superoxide dismutase and oxygen toxicity defenses in the genus Neisseria. Infect. Immun. 1986, 51, 631–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seib, K.L.; Jennings, M.P.; McEwan, A.G. A Sco homologue plays a role in defence against oxidative stress in pathogenic Neisseria. FEBS Lett. 2003, 546, 411–415. [Google Scholar] [CrossRef]
- Stohl, E.A.; Seifert, H.S. Neisseria gonorrhoeae DNA recombination and repair enzymes protect against oxidative damage caused by hydrogen peroxide. J. Bacteriol. 2006, 188, 7645–7651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, K.A.; Seifert, H.S. Mutation of the PriA gene of Neisseria gonorrhoeae affects DNA transformation and DNA repair. J. Bacteriol. 2005, 187, 5347–5355. [Google Scholar] [CrossRef] [Green Version]
- LeCuyer, B.E.; Criss, A.K.; Seifert, H.S. Genetic characterization of the nucleotide excision repair system of Neisseria gonorrhoeae. J. Bacteriol. 2010, 192, 665–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, M.B.; Criss, A.K. Resistance of Neisseria gonorrhoeae to neutrophils. Front. Microbiol. 2011, 2, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zughaier, S.M.; Kandler, J.L.; Shafer, W.M. Neisseria gonorrhoeae modulates iron-limiting innate immune defenses in macrophages. PLoS ONE 2014, 9, e87688. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.C.; Lefimil, C.; Rodas, P.I.; Vernal, R.; Lopez, M.; Acuña-Castillo, C.; Imarai, M.; Escobar, A. Neisseria gonorrhoeae modulates immunity by polarizing human macrophages to a M2 profile. PLoS ONE 2015, 10, e0130713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerse, A.E.; Sharma, N.D.; Simms, A.N.; Crow, E.T.; Snyder, L.A.; Shafer, W.M. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect. Immun. 2003, 71, 5576–5582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handing, J.W.; Ragland, S.A.; Bharathan, U.V.; Criss, A.K. The MtrCDE efflux pump contributes to survival of Neisseria gonorrhoeae from human neutrophils and their antimicrobial components. Front. Microbiol. 2018, 9, 2688. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-G.; Zhao, Y.; Zhu, D.; Gillings, M.; Penuelas, J.; Ok, Y.S.; Capon, A.; Banwart, S. Soil biota, antimicrobial resistance and planetary health. Environ. Int. 2019, 131, 105059. [Google Scholar] [CrossRef]
- Hamilton, H.L.; Dillard, J.P. Natural transformation of Neisseria gonorrhoeae: From DNA donation to homologous recombination. Mol. Microbiol. 2006, 59, 376–385. [Google Scholar] [CrossRef]
- Unemo, M.; Shafer, W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: Past, evolution, and future. Clin. Microbiol. Rev. 2014, 27, 587–613. [Google Scholar] [CrossRef] [Green Version]
- Palace, S.G.; Wang, Y.; Rubin, D.H.; Welsh, M.A.; Mortimer, T.D.; Cole, K.; Eyre, D.W.; Walker, S.; Grad, Y.H. RNA polymerase mutations cause cephalosporin resistance in clinical Neisseria gonorrhoeae isolates. eLife 2020, 9, e51407. [Google Scholar] [CrossRef] [PubMed]
- Dickey, S.W.; Cheung, G.Y.C.; Otto, M. Different drugs for bad bugs: Antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 2017, 16, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Foschi, C.; Salvo, M.; Cevenini, R.; Parolin, C.; Vitali, B.; Marangoni, A. Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: An in vitro study. Front. Cell. Infect. Microbiol. 2017, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.D.; Wright, J.C.; Li, J.; Hood, D.W.; Moxon, E.R.; Richards, J.C. Phosphorylation of the lipid A region of meningococcal lipopolysaccharide: Identification of a family of transferases that add phosphoethanolamine to lipopolysaccharide. J. Bacteriol. 2003, 185, 3270–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, L.A.; Choudhury, B.; Balthazar, J.T.; Martin, L.E.; Ram, S.; Rice, P.A.; Stephens, D.S.; Carlson, R.; Shafer, W.M. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect. Immun. 2009, 77, 1112–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, M.M.; Anderson, J.E.; Balthazar, J.T.; Kandler, J.L.; Carlson, R.W.; Ganguly, J.; Begum, A.A.; Duncan, J.A.; Lin, J.T.; Sparling, P.F.; et al. Lipid A’s structure mediates Neisseria gonorrhoeae fitness during experimental infection of mice and men. mBio 2013, 4, e00892-13. [Google Scholar] [CrossRef] [Green Version]
- Handing, J.W.; Criss, A.K. The lipooligosaccharide-modifying enzyme LptA enhances gonococcal defence against human neutrophils. Cell. Microbiol. 2015, 17, 910–921. [Google Scholar] [CrossRef] [Green Version]
- Anandan, A.; Evans, G.L.; Condic-Jurkic, K.; O’Mara, M.L.; John, C.M.; Phillips, N.J.; Jarvis, G.A.; Wills, S.S.; Stubbs, K.A.; Moraes, I.; et al. Structure of a lipid A phosphoethanolamine transferase suggests how conformational changes govern substrate binding. Proc. Natl. Acad. Sci. USA 2017, 114, 2218–2223. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Jerse, A.E. α-2,3-sialyltransferase enhances Neisseria gonorrhoeae survival during experimental murine genital tract infection. Infect. Immun. 2006, 74, 4094–4103. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.Y.-C.; Rakic, B.; Chiu, C.P.C.; Lameignere, E.; Wakarchuk, W.W.; Withers, S.G.; Strynadka, N.C.J. Structure and mechanism of the lipooligosaccharide sialyltransferase from Neisseria meningitidis. J. Biol. Chem. 2011, 286, 37237–37248. [Google Scholar] [CrossRef] [Green Version]
- Lewis, L.A.; Gulati, S.; Burrowes, E.; Zheng, B.; Ram, S.; Rice, P.A. α-2,3-sialyltransferase expression level impacts the kinetics of lipooligosaccharide sialylation, complement resistance, and the ability of Neisseria gonorrhoeae to colonize the murine genital tract. mBio 2015, 6, e02465-14. [Google Scholar] [CrossRef] [Green Version]
- Gulati, S.; Schoenhofen, I.C.; Whitfield, D.M.; Cox, A.D.; Li, J.; Michael, F.S.; Vinogradov, E.V.; Stupak, J.; Zheng, B.; Ohnishi, M.; et al. Utilizing CMP-sialic acid analogs to unravel Neisseria gonorrhoeae lipooligosaccharide-mediated complement resistance and design novel therapeutics. PLoS Pathog. 2015, 11, e1005290. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, J.; Gulati, S.; Agarwal, S.; Unemo, M.; Ohnishi, M.; Su, X.-H.; Monks, B.G.; Visintin, A.; Madico, G.; Lewis, L.A.; et al. A novel factor H–Fc chimeric immunotherapeutic molecule against Neisseria gonorrhoeae. J. Immunol. 2016, 196, 1732–1740. [Google Scholar] [CrossRef] [Green Version]
- Gulati, S.; Schoenhofen, I.C.; Lindhout-Djukic, T.; Schur, M.J.; Landig, C.S.; Saha, S.; Deng, L.; Lewis, L.A.; Zheng, B.; Varki, A.; et al. Therapeutic CMP-nonulosonates against multidrug-resistant Neisseria gonorrhoeae. J. Immunol. 2020, 204, 3283–3295. [Google Scholar] [CrossRef]
- Gulati, S.; Schoenhofen, I.C.; Lindhout-Djukic, T.; Lewis, L.A.; Moustafa, I.Y.; Saha, S.; Zheng, B.; Nowak, N.; Rice, P.A.; Varki, A.; et al. Efficacy of antigonococcal CMP-nonulosonate therapeutics require cathelicidins. J. Infect. Dis. 2020, 222, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.; Kim, M.J.; Xu, Y.; Jin, X.L.; Yoo, H.Y.; Park, J.-W.; Gong, J.H.; Choe, K.-M.; Lee, B.L.; Ha, N.-C. Structural basis for the recognition of lysozyme by MliC, a periplasmic lysozyme inhibitor in Gram-negative bacteria. Biochem. Biophys. Res. Commun. 2009, 378, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Ragland, S.A.; Humbert, M.V.; Christodoulides, M.; Criss, A.K. Neisseria gonorrhoeae employs two protein inhibitors to evade killing by human lysozyme. PLoS Pathog. 2018, 14, e1007080. [Google Scholar] [CrossRef] [PubMed]
- Zielke, R.A.; Le Van, A.; Baarda, B.I.; Herrera, M.F.; Acosta, C.J.; Jerse, A.E.; Sikora, A.E. SliC is a surface-displayed lipoprotein that is required for the anti-lysozyme strategy during Neisseria gonorrhoeae infection. PLoS Pathog. 2018, 14, e1007081. [Google Scholar] [CrossRef]
- Almonacid-Mendoza, H.L.; Humbert, M.V.; Dijokaite, A.; Cleary, D.W.; Soo, Y.; Hung, M.-C.; Orr, C.M.; Machelett, M.M.; Tews, I.; Christodoulides, M. Structure of the recombinant Neisseria gonorrhoeae adhesin complex protein (rNg-ACP) and generation of murine antibodies with bactericidal activity against gonococci. mSphere 2018, 3, e00331-18. [Google Scholar] [CrossRef] [Green Version]
- Blundell, J.K.; Smith, G.J.; Perkins, H.R. The peptidoglycan of Neisseria gonorrhoeae: O-acetyl groups and lysozyme sensitivity. FEMS Microbiol. Lett. 1980, 9, 259–261. [Google Scholar] [CrossRef]
- Rosenthal, R.S.; Folkening, W.J.; Miller, D.R.; Swim, S.C. Resistance of O-acetylated gonococcal peptidoglycan to human peptidoglycan-degrading enzymes. Infect. Immun. 1983, 40, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, A.J.; Dupont, C. O-Acetylated peptidoglycan: Its occurrence, pathobiological significance, and biosynthesis. Can. J. Microbiol. 1992, 38, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Weadge, J.T.; Pfeffer, J.M.; Clarke, A.J. Identification of a new family of enzymes with potential O-acetylpeptidoglycan esterase activity in both Gram-positive and Gram-negative bacteria. BMC Microbiol. 2005, 5, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moynihan, P.J.; Clarke, A.J. O-acetylation of peptidoglycan in gram-negative bacteria. Identification and characterization of peptidoglycan O-acetyltransferase in Neisseria gonorrhoeae. J. Biol. Chem. 2010, 285, 13264–13273. [Google Scholar] [CrossRef] [Green Version]
- Brott, A.S.; Jones, C.S.; Clarke, A.J. Development of a high throughput screen for the identification of inhibitors of peptidoglycan O-acetyltransferases, new potential antibacterial targets. Antibiotics 2019, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Brott, A.S.; Clarke, A.J. Peptidoglycan O-acetylation as a virulence factor: Its effect on lysozyme in the innate immune system. Antibiotics 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.S.; Sychantha, D.; Howell, P.L.; Clarke, A.J. Structural basis for the O-acetyltransferase function of the extracytoplasmic domain of OatA from Staphylococcus aureus. J. Biol. Chem. 2020, 295, 8204–8213. [Google Scholar] [CrossRef]
- Cloud, K.A.; Dillard, J.P. A lytic transglycosylase of Neisseria gonorrhoeae is involved in peptidoglycan-derived cytotoxin production. Infect. Immun. 2002, 70, 2752–2757. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.A.; Hackett, K.T.; Dillard, J.P. The lytic transglycosylases of Neisseria gonorrhoeae. Microb. Drug Resist. 2012, 18, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Schaub, R.E.; Chan, Y.A.; Lee, M.; Hesek, D.; Mobashery, S.; Dillard, J.P. Lytic transglycosylases LtgA and LtgD perform distinct roles in remodeling, recycling and releasing peptidoglycan in Neisseria gonorrhoeae. Mol. Microbiol. 2016, 102, 865–881. [Google Scholar] [CrossRef] [Green Version]
- Ragland, S.A.; Schaub, R.E.; Hackett, K.T.; Dillard, J.P.; Criss, A.K. Two lytic transglycosylases in Neisseria gonorrhoeae impart resistance to killing by lysozyme and human neutrophils. Cell. Microbiol. 2017, 19, e12662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.H.; Wheeler, R.; Thiriau, C.; Haouz, A.; Taha, M.-K.; Boneca, I.G. Bulgecin A: The key to a broad-spectrum inhibitor that targets lytic transglycosylases. Antibiotics 2017, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.H.; Wheeler, R.; Deghmane, A.-E.; Santecchia, I.; Schaub, R.E.; Hicham, S.; Moya Nilges, M.; Malosse, C.; Chamot-Rooke, J.; Haouz, A.; et al. Defective lytic transglycosylase disrupts cell morphogenesis by hindering cell wall de-O-acetylation in Neisseria meningitidis. eLife 2020, 9, e51247. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, M.J.; Murphy, M.E.P. Crystal structure of the soluble domain of the major anaerobically induced outer membrane protein (AniA) from pathogenic Neisseria: A new class of copper-containing nitrite reductases. J. Mol. Biol. 2002, 315, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Falsetta, M.L.; Bair, T.B.; Ku, S.C.; vanden Hoven, R.N.; Steichen, C.T.; McEwan, A.G.; Jennings, M.P.; Apicella, M.A. Transcriptional profiling identifies the metabolic phenotype of gonococcal biofilms. Infect. Immun. 2009, 77, 3522–3532. [Google Scholar] [CrossRef] [Green Version]
- Shewell, L.K.; Ku, S.C.; Schulz, B.L.; Jen, F.E.-C.; Mubaiwa, T.D.; Ketterer, M.R.; Apicella, M.A.; Jennings, M.P. Recombinant truncated AniA of pathogenic Neisseria elicits a non-native immune response and functional blocking antibodies. Biochem. Biophys. Res. Commun. 2013, 431, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Sikora, A.E.; Mills, R.H.; Weber, J.V.; Hamza, A.; Passow, B.W.; Romaine, A.; Williamson, Z.A.; Reed, R.W.; Zielke, R.A.; Korotkov, K.V. Peptide inhibitors targeting the Neisseria gonorrhoeae pivotal anaerobic respiration factor AniA. Antimicrob. Agents Chemother. 2017, 61, e00186-17. [Google Scholar] [CrossRef] [Green Version]
- Sikora, A.; Rotkov, K.V. Peptide Inhibitors Targeting the Neisseria gonorrhoeae Pivotal Anaerobic Respiration Factor AniA. 2019. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=US279743297 (accessed on 5 September 2020).
- Lomovskaya, O.; Warren, M.S.; Lee, A.; Galazzo, J.; Fronko, R.; Lee, M.; Blais, J.; Cho, D.; Chamberland, S.; Renau, T.; et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob. Agents Chemother. 2001, 45, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Akama, H.; Matsuura, T.; Kashiwagi, S.; Yoneyama, H.; Narita, S.; Tsukihara, T.; Nakagawa, A.; Nakae, T. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J. Biol. Chem. 2004, 279, 25939–25942. [Google Scholar] [CrossRef] [Green Version]
- Lomovskaya, O.; Bostian, K.A. Practical applications and feasibility of efflux pump inhibitors in the clinic—A vision for applied use. Biochem. Pharmacol. 2006, 71, 910–918. [Google Scholar] [CrossRef]
- Lei, H.-T.; Chou, T.-H.; Su, C.-C.; Bolla, J.R.; Kumar, N.; Radhakrishnan, A.; Long, F.; Delmar, J.A.; Do, S.V.; Rajashankar, K.R.; et al. Crystal structure of the open state of the Neisseria gonorrhoeae MtrE outer membrane channel. PLoS ONE 2014, 9, e97475. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Connolly, K.L.; Rouquette-Loughlin, C.; D’Andrea, A.; Jerse, A.E.; Shafer, W.M. Could dampening expression of the Neisseria gonorrhoeae mtrCDE-encoded efflux pump be a strategy to preserve currently or resurrect formerly used antibiotics to treat gonorrhea? mBio 2019, 10, e01576-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, M.; Moseng, M.A.; Reimche, J.L.; Holley, C.L.; Dhulipala, V.; Su, C.-C.; Shafer, W.M.; Yu, E.W. Cryo-EM structures of a gonococcal multidrug efflux pump illuminate a mechanism of drug recognition and resistance. mBio 2020, 11, e00996-20. [Google Scholar] [CrossRef] [PubMed]
- Riboldi-Tunnicliffe, A.; König, B.; Jessen, S.; Weiss, M.S.; Rahfeld, J.; Hacker, J.; Fischer, G.; Hilgenfeld, R. Crystal structure of Mip, a prolylisomerase from Legionella pneumophila. Nat. Struct. Biol. 2001, 8, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Leuzzi, R.; Serino, L.; Scarselli, M.; Savino, S.; Fontana, M.R.; Monaci, E.; Taddei, A.; Fischer, G.; Rappuoli, R.; Pizza, M. Ng-MIP, a surface-exposed lipoprotein of Neisseria gonorrhoeae, has a peptidyl-prolyl cis/trans isomerase (PPIase) activity and is involved in persistence in macrophages. Mol. Microbiol. 2005, 58, 669–681. [Google Scholar] [CrossRef]
- Reimer, A.; Seufert, F.; Weiwad, M.; Ebert, J.; Bzdyl, N.M.; Kahler, C.M.; Sarkar-Tyson, M.; Holzgrabe, U.; Rudel, T.; Kozjak-Pavlovic, V. Inhibitors of macrophage infectivity potentiator-like PPIases affect neisserial and chlamydial pathogenicity. Int. J. Antimicrob. Agents 2016, 48, 401–408. [Google Scholar] [CrossRef]
- Bardwell, J.C.; Lee, J.-O.; Jander, G.; Martin, N.; Belin, D.; Beckwith, J. A pathway for disulfide bond formation in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 1038–1042. [Google Scholar] [CrossRef] [Green Version]
- Missiakas, D.; Georgopoulos, C.; Raina, S. Identification and characterization of the Escherichia coli gene dsbB, whose product is involved in the formation of disulfide bonds in vivo. Proc. Natl. Acad. Sci. USA 1993, 90, 7084–7088. [Google Scholar] [CrossRef] [Green Version]
- Tinsley, C.R.; Voulhoux, R.; Beretti, J.-L.; Tommassen, J.; Nassif, X. Three homologues, including two membrane-bound proteins, of the disulfide oxidoreductase DsbA in Neisseria meningitidis: Effects on bacterial growth and biogenesis of functional type IV pili. J. Biol. Chem. 2004, 279, 27078–27087. [Google Scholar] [CrossRef] [Green Version]
- Miki, T.; Okada, N.; Danbara, H. Two periplasmic disulfide oxidoreductases, DsbA and SrgA, target outer membrane protein SpiA, a component of the Salmonella pathogenicity island 2 type III secretion system. J. Biol. Chem. 2004, 279, 34631–34642. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Ambur, O.H.; Langford, P.R.; Tønjum, T.; Kroll, J.S. Reduced DNA binding and uptake in the absence of DsbA1 and DsbA2 of Neisseria meningitidis due to inefficient folding of the outer-membrane secretin PilQ. Microbiology 2008, 154, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malojčić, G.; Owen, R.L.; Grimshaw, J.P.A.; Glockshuber, R. Preparation and structure of the charge-transfer intermediate of the transmembrane redox catalyst DsbB. FEBS Lett. 2008, 582, 3301–3307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totsika, M.; Heras, B.; Wurpel, D.J.; Schembri, M.A. Characterization of two homologous disulfide bond systems involved in virulence factor biogenesis in uropathogenic Escherichia coli CFT073. J. Bacteriol. 2009, 191, 3901–3908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafaye, C.; Iwema, T.; Carpentier, P.; Jullian-Binard, C.; Kroll, J.S.; Collet, J.-F.; Serre, L. Biochemical and structural study of the homologues of the thiol–disulfide oxidoreductase DsbA in Neisseria meningitidis. J. Mol. Biol. 2009, 392, 952–966. [Google Scholar] [CrossRef]
- Vivian, J.P.; Scoullar, J.; Rimmer, K.; Bushell, S.R.; Beddoe, T.; Wilce, M.C.J.; Byres, E.; Boyle, T.P.; Doak, B.; Simpson, J.S.; et al. Structure and function of the oxidoreductase DsbA1 from Neisseria meningitidis. J. Mol. Biol. 2009, 394, 931–943. [Google Scholar] [CrossRef]
- Früh, V.; Zhou, Y.; Chen, D.; Loch, C.; Eiso, A.; Grinkova, Y.N.; Verheij, H.; Sligar, S.G.; Bushweller, J.H.; Siegal, G. Application of fragment based drug discovery to membrane proteins: Biophysical identification of ligands of the integral membrane enzyme DsbB. Chem. Biol. 2010, 17, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Ireland, P.M.; McMahon, R.M.; Marshall, L.E.; Halili, M.; Furlong, E.; Tay, S.; Martin, J.L.; Sarkar-Tyson, M. Disarming Burkholderia pseudomallei: Structural and functional characterization of a disulfide oxidoreductase (DsbA) required for virulence in vivo. Antioxid. Redox Signal. 2014, 20, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.A.; Sharma, P.; Mohanty, B.; Ilyichova, O.V.; Mulcair, M.D.; Williams, M.L.; Gleeson, E.C.; Totsika, M.; Doak, B.C.; Caria, S.; et al. Application of fragment-based screening to the design of inhibitors of Escherichia coli DsbA. Angew. Chem. Int. Ed. 2015, 54, 2179–2184. [Google Scholar] [CrossRef]
- Landeta, C.; Blazyk, J.L.; Hatahet, F.; Meehan, B.M.; Eser, M.; Myrick, A.; Bronstain, L.; Minami, S.; Arnold, H.; Ke, N.; et al. Compounds targeting disulfide bond forming enzyme DsbB of Gram-negative bacteria. Nat. Chem. Biol. 2015, 11, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Halili, M.A.; Bachu, P.; Lindahl, F.; Bechara, C.; Mohanty, B.; Reid, R.C.; Scanlon, M.J.; Robinson, C.V.; Fairlie, D.P.; Martin, J.L. Small molecule inhibitors of disulfide bond formation by the bacterial DsbA-DsbB dual enzyme system. ACS Chem. Biol. 2015, 10, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.P.; Paxman, J.J.; Scanlon, M.J.; Heras, B. Targeting bacterial Dsb proteins for the development of anti-virulence agents. Molecules 2016, 21, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, R.M.; Ireland, P.M.; Sarovich, D.S.; Petit, G.; Jenkins, C.H.; Sarkar-Tyson, M.; Currie, B.J.; Martin, J.L. Virulence of the melioidosis pathogen Burkholderia pseudomallei requires the oxidoreductase membrane protein DsbB. Infect. Immun. 2018, 86, e00938-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totsika, M.; Vagenas, D.; Paxman, J.J.; Wang, G.; Dhouib, R.; Sharma, P.; Martin, J.L.; Scanlon, M.J.; Heras, B. Inhibition of diverse DsbA enzymes in multi-DsbA encoding pathogens. Antioxid. Redox Signal. 2018, 29, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Duncan, L.F.; Wang, G.; Ilyichova, O.V.; Scanlon, M.J.; Heras, B.; Abbott, B.M. The fragment-based development of a benzofuran hit as a new class of Escherichia coli DsbA inhibitors. Molecules 2019, 24, 3756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellogg, D.S.; Cohen, I.R.; Norins, L.C.; Schroeter, A.L.; Reising, G. Neisseria gonorrhoeae II. Colonial variation and pathogenicity during 35 months in vitro. J. Bacteriol. 1968, 96, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Rudel, T.; van Putten, J.P.M.; Gibbs, C.P.; Haas, R.; Meyer, T.F. Interaction of two variable proteins (PilE and PilC) required for pilus-mediated adherence of Neisseria gonorrhoeae to human epithelial cells. Mol. Microbiol. 1992, 6, 3439–3450. [Google Scholar] [CrossRef] [PubMed]
- Seifert, H.S.; Wright, C.J.; Jerse, A.E.; Cohen, M.S.; Cannon, J.G. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Investig. 1994, 93, 2744–2749. [Google Scholar] [CrossRef] [Green Version]
- Rudel, T.; Scheuerpflug, I.; Meyer, T.F. Neisseria PilC protein identified as type-4 pilus tip-located adhesin. Nature 1995, 373, 357–359. [Google Scholar] [CrossRef]
- Parge, H.E.; Forest, K.T.; Hickey, M.J.; Christensen, D.A.; Getzoff, E.D.; Tainer, J.A. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature 1995, 378, 32–38. [Google Scholar] [CrossRef]
- Wolfgang, M.; Lauer, P.; Park, H.-S.; Brossay, L.; Hébert, J.; Koomey, M. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 1998, 29, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Merz, A.J.; So, M.; Sheetz, M.P. Pilus retraction powers bacterial twitching motility. Nature 2000, 407, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Winther-Larsen, H.C.; Wolfgang, M.; Dunham, S.; Putten, J.P.M.V.; Dorward, D.; Løvold, C.; Aas, F.E.; Koomey, M. A conserved set of pilin-like molecules controls type IV pilus dynamics and organelle-associated functions in Neisseria gonorrhoeae. Mol. Microbiol. 2005, 56, 903–917. [Google Scholar] [CrossRef]
- Craig, L.; Volkmann, N.; Arvai, A.S.; Pique, M.E.; Yeager, M.; Egelman, E.H.; Tainer, J.A. Type IV pilus structure by cryo-electron microscopy and crystallography: Implications for pilus assembly and functions. Mol. Cell 2006, 23, 651–662. [Google Scholar] [CrossRef]
- Hobbs, M.M.; Sparling, P.F.; Cohen, M.S.; Shafer, W.M.; Deal, C.D.; Jerse, A.E. Experimental gonococcal infection in male volunteers: Cumulative experience with Neisseria gonorrhoeae strains FA1090 and MS11mkc. Front. Microbiol. 2011, 2, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Coureuil, M.; Osinski, T.; Orlova, A.; Altindal, T.; Gesbert, G.; Nassif, X.; Egelman, E.H.; Craig, L. Cryoelectron microscopy reconstructions of the Pseudomonas aeruginosa and Neisseria gonorrhoeae type IV pili at sub-nanometer resolution. Structure 2017, 25, 1423–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubey, F.; Corre, J.-P.; Kong, Y.; Xu, X.; Obino, D.; Goussard, S.; Lapeyrere, C.; Souphron, J.; Couturier, C.; Renard, S.; et al. Inhibitors of the Neisseria meningitidis PilF ATPase provoke type IV pilus disassembly. Proc. Natl. Acad. Sci. USA 2019, 116, 8481–8486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denis, K.; Le Bris, M.; Le Guennec, L.; Barnier, J.-P.; Faure, C.; Gouge, A.; Bouzinba-Ségard, H.; Jamet, A.; Euphrasie, D.; Durel, B.; et al. Targeting type IV pili as an antivirulence strategy against invasive meningococcal disease. Nat. Microbiol. 2019, 4, 972–984. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.; Day, C.J.; Haselhorst, T.; Jen, F.E.-C.; Torres, V.J.; Edwards, J.L.; Jennings, M.P. Repurposed drugs that block the gonococcus-complement receptor 3 interaction can prevent and cure gonococcal infection of primary human cervical epithelial cells. mBio 2020, 11, e03046-19. [Google Scholar] [CrossRef] [Green Version]
- Swanson, J.; Barrera, O.; Sola, J.; Boslego, J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J. Exp. Med. 1988, 168, 2121–2129. [Google Scholar] [CrossRef] [Green Version]
- Jerse, A.E.; Cohen, M.S.; Drown, P.M.; Whicker, L.G.; Isbey, S.F.; Seifert, H.S.; Cannon, J.G. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J. Exp. Med. 1994, 179, 911–920. [Google Scholar] [CrossRef]
- Schneider, H.; Cross, A.S.; Kuschner, R.A.; Taylor, D.N.; Sadoff, J.C.; Boslego, J.W.; Deal, C.D. Experimental human gonococcal urethritis: 250 Neisseria gonorrhoeae MS11mkC are infective. J. Infect. Dis. 1995, 172, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.C.; Bos, M.P.; Belland, R.J. Proteoglycan receptor binding by Neisseria gonorrhoeae MS11 is determined by the HV-1 region of OpaA. Mol. Microbiol. 1999, 32, 233–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, K.A.; Deal, C.D.; Kwan, M.; Thattassery, E.; Schneider, H. Neisseria gonorrhoeae MS11mkC opacity protein expression in vitro and during human volunteer infectivity studies. Sex. Transm. Dis. 2000, 27, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, D.A.; Larsson, P.; Lo, R.H.; Kroncke, B.M.; Kasson, P.M.; Columbus, L. Structure of the neisserial outer membrane protein Opa60: Loop flexibility essential to receptor recognition and bacterial engulfment. J. Am. Chem. Soc. 2014, 136, 9938–9946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semchenko, E.A.; Everest-Dass, A.V.; Jen, F.E.-C.; Mubaiwa, T.D.; Day, C.J.; Seib, K.L. Glycointeractome of Neisseria gonorrhoeae: Identification of host glycans targeted by the gonococcus to facilitate adherence to cervical and urethral epithelial cells. mBio 2019, 10, e01339-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Osborn, M.J. Structure and biosynthesis of the bacterial cell wall. Annu. Rev. Biochem. 1969, 38, 501–538. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Kandler, O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 1972, 36, 407–477. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; Pedro, M.A.D. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preston, A.; Mandrell, R.E.; Gibson, B.W.; Apicella, M.A. The lipooligosaccharides of pathogenic Gram-negative bacteria. Crit. Rev. Microbiol. 1996, 22, 139–180. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Qureshi, N.; Hyver, K.; Honovich, J.; Cotter, R.J.; Mascagni, P.; Schneider, H. Characterization of a structural series of lipid A obtained from the lipopolysaccharides of Neisseria gonorrhoeae. Combined laser desorption and fast atom bombardment mass spectral analysis of high performance liquid chromatography-purified dimethyl derivatives. J. Biol. Chem. 1986, 261, 10624–10631. [Google Scholar] [PubMed]
- Kulshin, V.A.; Zähringer, U.; Lindner, B.; Frasch, C.E.; Tsai, C.M.; Dmitriev, B.A.; Rietschel, E.T. Structural characterization of the lipid A component of pathogenic Neisseria meningitidis. J. Bacteriol. 1992, 174, 1793–1800. [Google Scholar] [CrossRef] [Green Version]
- Dillard, J.P. Peptidoglycan metabolism and fragment production. In Pathogenic Neisseria: Genomics, Molecular Biology and Disease Intervention; Davies, J.K., Kahler, C.M., Eds.; Caister Academic Press: Norfolk, UK, 2014; pp. 97–114. ISBN 978-1-908230-61-4. [Google Scholar]
- Wanty, C.; Anandan, A.; Piek, S.; Walshe, J.; Ganguly, J.; Carlson, R.W.; Stubbs, K.A.; Kahler, C.M.; Vrielink, A. The structure of the neisserial lipooligosaccharide phosphoethanolamine transferase A (LptA) required for resistance to polymyxin. J. Mol. Biol. 2013, 425, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- John, C.M.; Liu, M.; Phillips, N.J.; Yang, Z.; Funk, C.R.; Zimmerman, L.I.; Griffiss, J.M.; Stein, D.C.; Jarvis, G.A. Lack of lipid A pyrophosphorylation and functional lptA reduces inflammation by Neisseria commensals. Infect. Immun. 2012, 80, 4014–4026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzeng, Y.-L.; Ambrose, K.D.; Zughaier, S.; Zhou, X.; Miller, Y.K.; Shafer, W.M.; Stephens, D.S. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 2005, 187, 5387–5396. [Google Scholar] [CrossRef] [Green Version]
- Kandler, J.L.; Joseph, S.J.; Balthazar, J.T.; Dhulipala, V.; Read, T.D.; Jerse, A.E.; Shafer, W.M. Phase-variable expression of lptA modulates the resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides. Antimicrob. Agents Chemother. 2014, 58, 4230–4233. [Google Scholar] [CrossRef] [Green Version]
- Kahler, C.M.; Nawrocki, K.L.; Anandan, A.; Vrielink, A.; Shafer, W.M. Structure-function relationships of the neisserial EptA enzyme responsible for phosphoethanolamine decoration of lipid A: Rationale for drug targeting. Front. Microbiol. 2018, 9, 1922. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, W.; Lei, S.; Lin, J.; Srinivas, S.; Feng, Y. An evolutionarily conserved mechanism for intrinsic and transferable polymyxin resistance. mBio 2018, 9, e0231-17. [Google Scholar] [CrossRef] [Green Version]
- Bartley, S.; Kahler, C.M. The glycome of Neisseria spp.: How does this relate to pathogenesis? In Pathogenic Neisseria: Genomics, Molecular Biology and Disease Intervention; Davies, J.K., Kahler, C.M., Eds.; Caister Academic Press: Norfolk, UK, 2014; pp. 115–145. ISBN 978-1-908230-61-4. [Google Scholar]
- Carbonnelle, E.; Hill, D.J.; Morand, P.; Griffiths, N.J.; Bourdoulous, S.; Murillo, I.; Nassif, X.; Virji, M. Meningococcal interactions with the host. Vaccine 2009, 27, B78–B89. [Google Scholar] [CrossRef]
- Parsons, N.J.; Andrade, J.R.C.; Patel, P.V.; Cole, J.A.; Smith, H. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5′-monophospho-N-acetyl neuraminic acid. Microb. Pathog. 1989, 7, 63–72. [Google Scholar] [CrossRef]
- Mandrell, R.E.; Lesse, A.J.; Sugai, J.V.; Shero, M.; Griffiss, J.M.; Cole, J.A.; Parsons, N.J.; Smith, H.; Morse, S.A.; Apicella, M.A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J. Exp. Med. 1990, 171, 1649–1664. [Google Scholar] [CrossRef] [Green Version]
- Wetzler, L.M.; Barry, K.; Blake, M.S.; Gotschlich, E.C. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect. Immun. 1992, 60, 39–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emond, J.-P.; Dublanchet, A.; Goldner, M. Kinetics of conversion of Neisseria gonorrhoeae to resistance to complement by cytidine 5′-monophospho-N-acetyl neuraminic acid. Antonie Van Leeuwenhoek 1995, 67, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Sharma, A.K.; Simpson, S.D.; Gulati, S.; McQuillen, D.P.; Pangburn, M.K.; Rice, P.A. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J. Exp. Med. 1998, 187, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devyatyarova-Johnson, M.; Rees, I.H.; Robertson, B.D.; Turner, M.W.; Klein, N.J.; Jack, D.L. The lipopolysaccharide structures of Salmonella enterica serovar Typhimurium and Neisseria gonorrhoeae determine the attachment of human mannose-binding lectin to intact organisms. Infect. Immun. 2000, 68, 3894–3899. [Google Scholar] [CrossRef] [Green Version]
- Gulati, S.; Sastry, K.; Jensenius, J.C.; Rice, P.A.; Ram, S. Regulation of the mannan-binding lectin pathway of complement on Neisseria gonorrhoeae by C1-inhibitor and α2-macroglobulin. J. Immunol. 2002, 168, 4078–4086. [Google Scholar] [CrossRef]
- Blom, A.M.; Hallström, T.; Riesbeck, K. Complement evasion strategies of pathogens—Acquisition of inhibitors and beyond. Mol. Immunol. 2009, 46, 2808–2817. [Google Scholar] [CrossRef]
- Mandrell, R.E.; Griffiss, J.M.; Smith, H.; Cole, J.A. Distribution of a lipooligosaccharide-specific sialyltransferase in pathogenic and non-pathogenic Neisseria. Microb. Pathog. 1993, 14, 315–327. [Google Scholar] [CrossRef]
- Matthias, K.A.; Rest, R.F. Control of pili and sialyltransferase expression in Neisseria gonorrhoeae is mediated by the transcriptional regulator CrgA. Mol. Microbiol. 2014, 91, 1120–1135. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Shaughnessy, J.; de Oliveira, R.B.; Lewis, L.A.; Gulati, S.; Rice, P.A. Gonococcal lipooligosaccharide sialylation: Virulence factor and target for novel immunotherapeutics. Pathog. Dis. 2017, 75, ftx049. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A. On a remarkable bacteriolytic element found in tissues and secretions. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character 1922, 93, 306–317. [Google Scholar] [CrossRef] [Green Version]
- Lelouard, H.; Henri, S.; De Bovis, B.; Mugnier, B.; Chollat–Namy, A.; Malissen, B.; Méresse, S.; Gorvel, J. Pathogenic bacteria and dead cells are internalized by a unique subset of Peyer’s patch dendritic cells that express lysozyme. Gastroenterology 2010, 138, 173–184. [Google Scholar] [CrossRef]
- Callewaert, L.; Michiels, C.W. Lysozymes in the animal kingdom. J. Biosci. 2010, 35, 127–160. [Google Scholar] [CrossRef]
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Epstein, L.A.; Chain, E. Some observations on the preparation and properties of the substrate of lysozyme. Br. J. Exp. Pathol. 1940, 21, 339–355. [Google Scholar]
- Salton, M.R.J. The properties of lysozyme and its action on microorganisms. Bacteriol. Rev. 1957, 21, 82–100. [Google Scholar] [CrossRef]
- McClure, R.; Nudel, K.; Massari, P.; Tjaden, B.; Su, X.; Rice, P.A.; Genco, C.A. The gonococcal transcriptome during infection of the lower genital tract in women. PLoS ONE 2015, 10, e0133982. [Google Scholar] [CrossRef] [Green Version]
- Humbert, M.V.; Awanye, A.M.; Lian, L.-Y.; Derrick, J.P.; Christodoulides, M. Structure of the Neisseria Adhesin Complex Protein (ACP) and its role as a novel lysozyme inhibitor. PLoS Pathog. 2017, 13, e1006448. [Google Scholar] [CrossRef]
- Dillard, J.P.; Hackett, K.T. Mutations affecting peptidoglycan acetylation in Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 2005, 73, 5697–5705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, B.H.; Rosenthal, R.S. Complement consumption gonococcal peptidoglycan. Infect. Immun. 1982, 35, 442–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, T.J.; Wallsmith, D.E.; Rosenthal, R.S. Arthropathic properties of gonococcal peptidoglycan fragments: Implications for the pathogenesis of disseminated gonococcal disease. Infect. Immun. 1986, 52, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Imada, A.; Kintaka, K.; Nakao, M.; Shinagawa, S. Bulgecin, a bacterial metabolite which in concert with β-lactam antibiotics causes bulge formation. J. Antibiot. 1982, 35, 1400–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinagawa, S.; Maki, M.; Kintaka, K.; Imada, A.; Asai, M. Isolation and characterization of bulgecins, new bacterial metabolites with bulge-inducing activity. J. Antibiot. 1985, 38, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thunnissen, A.-M.W.H.; Rozeboom, H.J.; Kalk, K.H.; Dijkstra, B.W. Structure of the 70-kDa soluble lytic transglycosylase complexed with bulgecin A. Implications for the enzymic mechanism. Biochemistry 1995, 34, 12729–12737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonis, M.; Williams, A.; Guadagnini, S.; Werts, C.; Boneca, I.G. The effect of bulgecin A on peptidoglycan metabolism and physiology of Helicobacter pylori. Microb. Drug Resist. 2012, 18, 230–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skalweit, M.J.; Li, M. Bulgecin A as a β-lactam enhancer for carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumannii clinical isolates containing various resistance mechanisms. Drug Des. Devel. Ther. 2016, 10, 3013–3020. [Google Scholar] [CrossRef] [Green Version]
- Dik, D.A.; Madukoma, C.S.; Tomoshige, S.; Kim, C.; Lastochkin, E.; Boggess, W.C.; Fisher, J.F.; Shrout, J.D.; Mobashery, S. Slt, MltD, and MltG of Pseudomonas aeruginosa as targets of Bulgecin A in potentiation of β-lactam antibiotics. ACS Chem. Biol. 2019, 14, 296–303. [Google Scholar] [CrossRef]
- Greiner, L.L.; Edwards, J.L.; Shao, J.; Rabinak, C.; Entz, D.; Apicella, M.A. Biofilm formation by Neisseria gonorrhoeae. Infect. Immun. 2005, 73, 1964–1970. [Google Scholar] [CrossRef] [Green Version]
- Steichen, C.T.; Shao, J.Q.; Ketterer, M.R.; Apicella, M.A. Gonococcal cervicitis: A role for biofilm in pathogenesis. J. Infect. Dis. 2008, 198, 1856–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, J.S.; Clark, V.L. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect. Immun. 1984, 46, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Householder, T.C.; Fozo, E.M.; Cardinale, J.A.; Clark, V.L. Gonococcal nitric oxide reductase is encoded by a single gene, norB, which is required for anaerobic growth and is induced by nitric oxide. Infect. Immun. 2000, 68, 5241–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, V.L.; Campbell, L.A.; Palermo, D.A.; Evans, T.M.; Klimpel, K.W. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect. Immun. 1987, 55, 1359–1364. [Google Scholar] [CrossRef] [Green Version]
- Clark, V.L.; Knapp, J.S.; Thompson, S.; Klimpel, K.W. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb. Pathog. 1988, 5, 381–390. [Google Scholar] [CrossRef]
- Li, X.-Z.; Nikaido, H. Efflux-mediated drug resistance in bacteria. Drugs 2004, 64, 159–204. [Google Scholar] [CrossRef]
- Hagman, K.E.; Pan, W.; Spratt, B.G.; Balthazar, J.T.; Judd, R.C.; Shafer, W.M. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 1995, 141, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Rouquette-Loughlin, C.E.; Balthazar, J.T.; Shafer, W.M. Characterization of the MacA–MacB efflux system in Neisseria gonorrhoeae. J. Antimicrob. Chemother. 2005, 56, 856–860. [Google Scholar] [CrossRef]
- Rouquette-Loughlin, C.; Dunham, S.A.; Kuhn, M.; Balthazar, J.T.; Shafer, W.M. The NorM efflux pump of Neisseria gonorrhoeae and Neisseria meningitidis recognizes antimicrobial cationic compounds. J. Bacteriol. 2003, 185, 1101–1106. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.H.; Shafer, W.M. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 1999, 33, 839–845. [Google Scholar] [CrossRef]
- Warner, D.M.; Shafer, W.M.; Jerse, A.E. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Mol. Microbiol. 2008, 70, 462–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadsworth, C.B.; Arnold, B.J.; Sater, M.R.A.; Grad, Y.H. Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. mBio 2018, 9, e01419-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouquette-Loughlin, C.E.; Reimche, J.L.; Balthazar, J.T.; Dhulipala, V.; Gernert, K.M.; Kersh, E.N.; Pham, C.D.; Pettus, K.; Abrams, A.J.; Trees, D.L.; et al. Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus. mBio 2018, 9, e02281-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delahay, R.M.; Robertson, B.D.; Balthazar, J.T.; Shafer, W.M.; Ison, C.A. Involvement of the gonococcal MtrE protein in the resistance of Neisseria gonorrhoeae to toxic hydrophobic agents. Microbiology 1997, 143, 2127–2133. [Google Scholar] [CrossRef] [Green Version]
- Hagman, K.E.; Lucas, C.E.; Balthazar, J.T.; Snyder, L.; Nilles, M.; Judd, R.C.; Shafer, W.M. The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 1997, 143, 2117–2125. [Google Scholar] [CrossRef] [Green Version]
- Pan, W.; Spratt, B.G. Regulation of the permeability of the gonococcal cell envelope by the mtr system. Mol. Microbiol. 1994, 11, 769–775. [Google Scholar] [CrossRef]
- Hagman, K.E.; Shafer, W.M. Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J. Bacteriol. 1995, 177, 4162–4165. [Google Scholar] [CrossRef] [Green Version]
- Rouquette, C.; Harmon, J.B.; Shafer, W.M. Induction of the mtrCDE-encoded efflux pump system of Neisseria gonorrhoeae requires MtrA, an AraC-like protein. Mol. Microbiol. 1999, 33, 651–658. [Google Scholar] [CrossRef]
- Shafer, W.M.; Qu, X.-D.; Waring, A.J.; Lehrer, R.I. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc. Natl. Acad. Sci. USA 1998, 95, 1829–1833. [Google Scholar] [CrossRef] [Green Version]
- Nudel, K.; McClure, R.; Moreau, M.; Briars, E.; Abrams, A.J.; Tjaden, B.; Su, X.-H.; Trees, D.; Rice, P.A.; Massari, P.; et al. Transcriptome analysis of Neisseria gonorrhoeae during natural infection reveals differential expression of antibiotic resistance determinants between men and women. mSphere 2018, 3, e00312-18. [Google Scholar] [CrossRef] [Green Version]
- Watkins, W.J.; Landaverry, Y.; Léger, R.; Litman, R.; Renau, T.E.; Williams, N.; Yen, R.; Zhang, J.Z.; Chamberland, S.; Madsen, D.; et al. The relationship between physicochemical properties, in vitro activity and pharmacokinetic profiles of analogues of diamine-containing efflux pump inhibitors. Bioorg. Med. Chem. Lett. 2003, 13, 4241–4244. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.; Villaverde, A. Protein quality in bacterial inclusion bodies. Trends Biotechnol. 2006, 24, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Hayer-Hartl, M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science 2002, 295, 1852–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, F.; Rinas, U. Roles of heat-shock chaperones in the production of recombinant proteins in Escherichia coli. Adv. Biochem. Eng. Biotechnol. 2004, 89, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Schiene-Fischer, C.; Yu, C. Receptor accessory folding helper enzymes: The functional role of peptidyl prolyl cis/trans isomerases. FEBS Lett. 2001, 495, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bardwell, J.C.A.; McGovern, K.; Beckwith, J. Identification of a protein required for disulfide bond formation in vivo. Cell 1991, 67, 581–589. [Google Scholar] [CrossRef]
- Brandts, J.F.; Halvorson, H.R.; Brennan, M. Consideration of the possibility that the slow step in protein denaturation reactions is due to cis-trans isomerism of proline residues. Biochemistry 1975, 14, 4953–4963. [Google Scholar] [CrossRef]
- Lang, K.; Schmid, F.X.; Fischer, G. Catalysis of protein folding by prolyl isomerase. Nature 1987, 329, 268–270. [Google Scholar] [CrossRef]
- Starnino, S.; Leuzzi, R.; Ghisetti, V.; De Francesco, M.A.; Cusini, M.; Impara, G.; Galluppi, E.; Pizza, M.; Stefanelli, P. Molecular analysis of two novel Neisseria gonorrhoeae virulent components: The macrophage infectivity potentiator and the outer membrane protein A. New Microbiol. 2010, 33, 167–170. [Google Scholar]
- Humbert, M.V.; Christodoulides, M. Immunization with recombinant truncated Neisseria meningitidis-Macrophage Infectivity Potentiator (rT-Nm-MIP) protein induces murine antibodies that are cross-reactive and bactericidal for Neisseria gonorrhoeae. Vaccine 2018, 36, 3926–3936. [Google Scholar] [CrossRef]
- Echenique-Rivera, H.; Muzzi, A.; Del Tordello, E.; Seib, K.L.; Francois, P.; Rappuoli, R.; Pizza, M.; Serruto, D. Transcriptome analysis of Neisseria meningitidis in human whole blood and mutagenesis studies identify virulence factors involved in blood survival. PLoS Pathog. 2011, 7, e1002027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bader, M.; Muse, W.; Ballou, D.P.; Gassner, C.; Bardwell, J.C.A. Oxidative protein folding is driven by the electron transport system. Cell 1999, 98, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Piek, S.; Wang, Z.; Ganguly, J.; Lakey, A.M.; Bartley, S.N.; Mowlaboccus, S.; Anandan, A.; Stubbs, K.A.; Scanlon, M.J.; Vrielink, A.; et al. The role of oxidoreductases in determining the function of the neisserial lipid A phosphoethanolamine transferase required for resistance to polymyxin. PLoS ONE 2014, 9, e106513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landeta, C.; Meehan, B.M.; McPartland, L.; Ingendahl, L.; Hatahet, F.; Tran, N.Q.; Boyd, D.; Beckwith, J. Inhibition of virulence-promoting disulfide bond formation enzyme DsbB is blocked by mutating residues in two distinct regions. J. Biol. Chem. 2017, 292, 6529–6541. [Google Scholar] [CrossRef] [Green Version]
- Winther-Larsen, H.; Hegge, F.; Wolfgang, M.; Hayes, S.; van Putten, J.; Koomey, M. Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence. Proc. Natl. Acad. Sci. USA 2001, 98, 15276–15281. [Google Scholar] [CrossRef] [Green Version]
- McGee, Z.A.; Johnson, A.P.; Taylor-Robinson, D. Pathogenic mechanisms of Neisseria gonorrhoeae: Observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J. Infect. Dis. 1981, 143, 413–422. [Google Scholar] [CrossRef]
- McGee, Z.A.; Stephens, D.S.; Hoffman, L.H.; Schlech, W.F.; Horn, R.G. Mechanisms of mucosal invasion by pathogenic Neisseria. Rev. Infect. Dis. 1983, 5, S708–S714. [Google Scholar] [CrossRef]
- Mosleh, I.M.; Boxberger, H.J.; Sessler, M.J.; Meyer, T.F. Experimental infection of native human ureteral tissue with Neisseria gonorrhoeae: Adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect. Immun. 1997, 65, 3391–3398. [Google Scholar] [CrossRef] [Green Version]
- Craig, L.; Pique, M.E.; Tainer, J.A. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2004, 2, 363–378. [Google Scholar] [CrossRef]
- Dietrich, M.; Bartfeld, S.; Munke, R.; Lange, C.; Ogilvie, L.A.; Friedrich, A.; Meyer, T.F. Activation of NF-κB by Neisseria gonorrhoeae is associated with microcolony formation and type IV pilus retraction. Cell. Microbiol. 2011, 13, 1168–1182. [Google Scholar] [CrossRef]
- Cahoon, L.A.; Seifert, H.S. Transcription of a cis-acting, noncoding, small RNA is required for pilin antigenic variation in Neisseria gonorrhoeae. PLoS Pathog. 2013, 9, e1003074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stohl, E.A.; Dale, E.M.; Criss, A.K.; Seifert, H.S. Neisseria gonorrhoeae metalloprotease NGO1686 is required for full piliation, and piliation is required for resistance to H2O2- and neutrophil-mediated killing. mBio 2013, 4, e00399-13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, J.-L.; Pelicic, V. Exceptionally widespread nanomachines composed of type IV pilins: The prokaryotic Swiss Army knives. FEMS Microbiol. Rev. 2015, 39, 134–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obergfell, K.P.; Seifert, H.S. The pilin N-terminal domain maintains Neisseria gonorrhoeae transformation competence during pilus phase variation. PLoS Genet. 2016, 12, e1006069. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Duarte, O.G.; Dehio, M.; Guzmán, C.A.; Chhatwal, G.S.; Dehio, C.; Meyer, T.F. Binding of vitronectin to opa-expressing Neisseria gonorrhoeae mediates invasion of HeLa cells. Infect. Immun. 1997, 65, 3857–3866. [Google Scholar] [CrossRef] [Green Version]
- Drake, S.L.; Koomey, M. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 1995, 18, 975–986. [Google Scholar] [CrossRef] [Green Version]
- Tønjum, T.; Caugant, D.A.; Dunham, S.A.; Koomey, M. Structure and function of repetitive sequence elements associated with a highly polymorphic domain of the Neisseria meningitidis PilQ protein. Mol. Microbiol. 1998, 29, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Lauer, P.; Albertson, N.H.; Koomey, M. Conservation of genes encoding components of a type IV pilus assembly/two-step protein export pathway in Neisseria gonorrhoeae. Mol. Microbiol. 1993, 8, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Wolfgang, M.; Putten, J.P.M.V.; Hayes, S.F.; Koomey, M. The comP locus of Neisseria gonorrhoeae encodes a type IV prepilin that is dispensable for pilus biogenesis but essential for natural transformation. Mol. Microbiol. 1999, 31, 1345–1357. [Google Scholar] [CrossRef] [Green Version]
- Freitag, N.E.; Seifert, H.S.; Koomey, M. Characterization of the pilF—pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol. Microbiol. 1995, 16, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Cole, J.G.; Fulcher, N.B.; Jerse, A.E. Opacity proteins increase Neisseria gonorrhoeae fitness in the female genital tract due to a factor under ovarian control. Infect. Immun. 2010, 78, 1629–1641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, M.L. A study of cervical cultures taken in cases of acute gonorrhea with special reference to the phases of the menstrual cycle. Am. J. Obstet. Gynecol. 1947, 54, 861–866. [Google Scholar] [CrossRef]
- Fleitas Martínez, O.; Cardoso, M.H.; Ribeiro, S.M.; Franco, O.L. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin neutralization, quorum-sensing interference and biofilm inhibition. Front. Cell. Infect. Microbiol. 2019, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Baldelli, V.; Halliday, N.; Pantalone, P.; Polticelli, F.; Fiscarelli, E.; Williams, P.; Visca, P.; Leoni, L.; Rampioni, G. Identification of FDA-approved drugs as antivirulence agents targeting the pqs quorum-sensing system of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2018, 62, e01296-18. [Google Scholar] [CrossRef] [Green Version]
- Rice, P.A.; Shafer, W.M.; Ram, S.; Jerse, A.E. Neisseria gonorrhoeae: Drug resistance, mouse models, and vaccine development. Annu. Rev. Microbiol. 2017, 71, 665–686. [Google Scholar] [CrossRef] [Green Version]
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Majed, A.; Abu-Hijleh, F.; Blondeel, K.; Matsaseng, T.C.; Kiarie, J.; Toskin, I.; Abu-Raddad, L.J. Global epidemiology of Neisseria gonorrhoeae in infertile populations: Systematic review, meta-analysis and metaregression. Sex. Transm. Infect. 2020. [Google Scholar] [CrossRef]
- Vallely, L.M.; Egli-Gany, D.; Wand, H.; Pomat, W.S.; Homer, C.S.E.; Guy, R.; Silver, B.; Rumbold, A.R.; Kaldor, J.M.; Vallely, A.J.; et al. Adverse pregnancy and neonatal outcomes associated with Neisseria gonorrhoeae: Systematic review and meta-analysis. Sex. Transm. Infect. 2021. [Google Scholar] [CrossRef]
- Balzarini, J.; Van Damme, L. Microbicide drug candidates to prevent HIV infection. Lancet 2007, 369, 787–797. [Google Scholar] [CrossRef]
- Musekiwa, A.; Fernando, N.B.; Abariga, S.A. Effectiveness of vaginal microbicides in preventing HIV transmission. Trop. Med. Int. Health 2020, 25, 790–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Slepenkin, A.; Elofsson, M.; Keyser, P.; de la Maza, L.M.; Peterson, E.M. Candidate vaginal microbicides with activity against Chlamydia trachomatis and Neisseria gonorrhoeae. Int. J. Antimicrob. Agents 2010, 36, 145–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, C.; Slepenkin, A.; Andersson, S.B.E.; Fagerberg, J.H.; Bergström, C.A.S.; Peterson, E.M. Formulation of the microbicide INP0341 for in vivo protection against a vaginal challenge by Chlamydia trachomatis. PLoS ONE 2014, 9, e110918. [Google Scholar] [CrossRef]
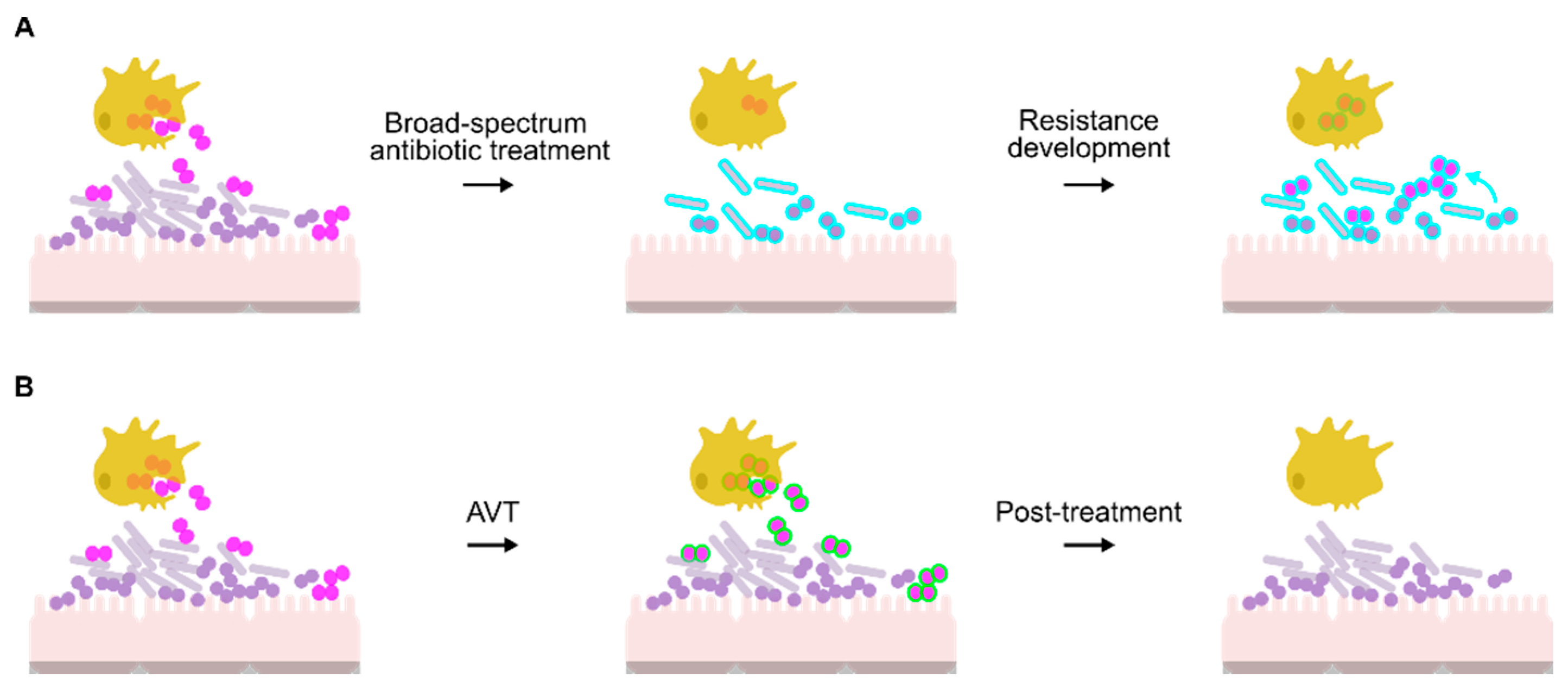

| Anti-Virulence Target | Function | Target Validation | Available Inhibitors | Inhibitor Studies | References | ||
|---|---|---|---|---|---|---|---|
| In Vitro Testing | Structural Studies | In Vivo Models | |||||
| Bacterial cell wall maintenance and modification | |||||||
| EptA | Catalyzes the addition of pEtN onto lipid A of the OM. | Loss of EptA increases susceptibility to killing by PMNs, macrophages, CAMPs and human serum. | Full structure solved of N. meningitidis homologue (98% identity, PDB accession code 5FGN). | Reduced survival rates of eptA mutant in mouse and human models. | N/A | N/A | [83,108,109,110,111,112] |
| Lst | Catalyzes the addition of N-acetyl-neuraminic acid onto lacto-N-neotetraose of LOS. Primary mechanism for resistance to human complement. | Loss of Lst increases susceptibility to killing by PMNs and human serum. | Full structure of N. meningitidis homologue apo form (92% identical, PDB accession code 2YK4 1) and with structural donor sugar analogs or products solved (PDB accession code 2YK5, 2YK6, and 2YK7 1). | Reduced survival rates of lst mutant in mouse models. | FHD1119G and Leg5,7Ac2 | Increased serum sensitivity. Significantly reduced duration and burden of infection in mouse vaginal colonization model. | [113,114,115,116,117,118,119] |
| NgACP and SliC | Essential for survival against lysozyme. | Loss of NgACP and SliC increased susceptibility to human lysozyme. NgACP loss significantly reduced survival in PMNs. | Mature NgACP structure has been solved (PDB accession code 6GQ4). Structure of SliC homologue in Pseudomonas aeruginosa (MliC) solved (23.3% identity, PDB accession code 3F6Z 1). | Reduced survival rates of sliC mutant in mouse models. | N/A | N/A | [120,121,122,123] |
| PatB | Catalyzes O-acetylation of N-acetyl-muramic acid. | Increased sensitivity to lysozyme in human sera or lysozyme purified from human PMNs. | Structure of PatB homologue in Staphylococcus aureus (OatA C-terminal catalytic domain) has been solved (15% identical, PDB accession code 6VJP 1). | N/A | Compound 89224 | Treatment reduced bacterial growth by 90%. Inhibitor binding studied using microtiter plate-based fluorometric assay. | [124,125,126,127,128,129,130,131] |
| LtgA | Catalyzes cleavage of N-acetyl-muramic acid-β-1,4-N-acetylglucosamine to form PG monomer fragments during cell growth. | Reduction in PG monomer release. Loss of LtgA in N. meningitidis has a detrimental effect on bacterial cell growth, division, and separation. | Structure of N. meningitidis homologue (97% identical, PDB accession code 6FPN 1). | NmLtgA mutant cleared quicker than wild-type and reduced cytokine induction in mouse model. | Bulgecin A | Inhibited LgtA activity and had a synergistic effect with β-lactams. | [75,132,133,134,135,136,137] |
| Anaerobic survival | |||||||
| AniA | Reduces nitrite to nitric oxide. Essential for anaerobic growth. | Loss of AniA reduces anaerobic growth and biofilm formation. | Soluble domain structure solved (PDB accession code 1KBW, 1KBV, 5TB7, and 5UE6). | Immunization with a truncated form of AniA generates protective antisera in a mouse model. | C7-3 | Significantly inhibited enzyme activity and gonococcal growth under anaerobic conditions. Inhibitor binding studied using molecular docking and biolayer interferometry. A patent has been approved for C7-3 and its derivatives. | [138,139,140,141,142] |
| Efflux pump | |||||||
| MtrCDE | Selective efflux of antimicrobials resulting in increased resistance to penicillins, macrolides and extended spectrum cephalosporins. | Loss of the MtrCDE pump results in increased susceptibility to penicillin, ceftriaxone, azithromycin, tetracycline, and solithromycin in the WHO clinical panel of multidrug-resistant (MDR) strains. | Full structures of MtrD and MtrE solved (PDB accession code 4MT1 (MtrD), 4MT0 (MtrE), 6VKS (MtrD from strain CR103 in complex with ampicillin) and 6VKT (MtrD from strain CR103 in complex with erythromycin). Full structure of MtrC homologue (MexA) in P. aeruginosa solved (43% identity, PDB accession code 1VF7 1). | Loss of the MtrCDE pump reduced gonococcal survival and increased penicillin susceptibility to therapeutic levels in mouse models. | Phenylalanine arginine β-naphthylamide (PaβN) | Untested in N. gonorrhoeae, derivatives have been halted due to high host cell toxicity. | [46,99,143,144,145,146,147,148] |
| Protein folding pathways | |||||||
| Mip | Catalyzes the cis–trans isomerization of peptide bonds directly preceding a proline residue. | Loss of Mip decreased gonococcal survival within macrophages and PMNs. | Full structure of Legionella pneumophila homologue solved (44.8% identical, PDB accession code 1FD9 1). | N/A | PipN3 and PipN4 | Compounds inhibited enzyme activity and reduce gonococcal survival in neutrophils. | [149,150,151] |
| DsbA/DsbB | Catalyzes formation of disulfide bonds in OM proteins involved in virulence. | Loss of DsbA1 in N. meningitidis affects tfp function, reducing colonization and competence. | Full structures of N. meningitidis homologues DsbA1 (97% identical, PDB accession code 3DVW and 3A3T 1) and DsbA3 (93% identical, PDB accession code 3DVX and 2ZNM1) solved. Full structure of E. coli DsbA/B complex homologue solved (28.7% identical, PDB accession code 3E9J 1). | N/A | Phenylthiazole, benzofuran, phenylthiophene or phenoxyphenyl derivatives | Untested in N. gonorrhoeae. | [152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169] |
| Adhesion and invasion | |||||||
| Type IV pili | Essential for adhesion/colonization, horizontal gene transfer, twitching motility. | Tfp mutants lacking PilE are unable to adhere to human epithelial cells, are non-motile and are incompetent. | Tfp structure has been solved (PDB accession code 5VXX, 1AY2, 2HIL and 2HI2). | In the human male model of infection, men inoculated with a gonococcal pilE mutant developed watery urethral discharge or were asymptomatic. | Compound B | Prevents pilus elongation. | [170,171,172,173,174,175,176,177,178,179,180,181,182,183] |
| Phenothiazines | Inhibited Na+-pumping NADH:quinone oxidoreductase. Tests with N. meningitidis reduced bacteremia and increased survival in a mouse model. | ||||||
| Compound G2, carbamazepine and methyldopa | Inhibits tfp binding to host receptor CR3 on primary cell line. Carbamazepine and methyldopa are re-purposed drug (FDA-approved anti-convulsant and high blood pressure medication, respectively). | ||||||
| Mannose-binding (Opa) proteins | Required for adherence to host epithelial cells. | Opa-less bacteria do not adhere to Chinese hamster ovary cells. | Structure of Opa60 has been solved (76% identity, PDB accession code 2MAF 1). | Gonococci recovered from human models are always Opa positive, even if the inoculum was Opa negative. | ConA and α-methyl D-mannoside (Mannosides) | Compounds reduced gonococcal adherence to primary cervical epithelial cells and urethral epithelial cells. | [184,185,186,187,188,189,190] |
| Characteristics | AVTs | Antibiotics | Hypothetical Vaccine |
|---|---|---|---|
| Mode of action | Selective inhibition of pathogens, preserves the microbiome | Broad spectrum killing of microorganisms, removes the microbiome | Selective inhibition of pathogen, preserves the microbiome |
| Mechanism of action | Tailored to prevent colonization, transmission, and infection by a pathogen | Kills systemic microorganisms—resolves acute infections. Not used for asymptomatic infections | Prevents acute infection by a pathogen. In some instances, vaccines can prevent colonization and transmission of the pathogen. |
| Use | Pre-exposure prophylaxis or therapeutic | Therapeutic | Pre-exposure therapeutic |
| Dose | Multiple dosing as needed | Multiple dosing, 3–4 days | 1–3 doses |
| Route of administration | Oral, topical  | Oral, injectable  | Injectable |
| Correlate of protection | Absence of viable pathogen at the site of infection | Absence of viable pathogen at the site of infection | Currently unknown |
| Implementation | Pharmacy or medical prescription | Medical prescription | Primary care clinics |
| Size and cost of clinical trials | >1000 subjects ** <$1 billion | >10,000 subjects >$1.5 billion | >100,000 subjects >$1.8 billion |
| Drug development timeframe from pre-clinical to licensing | 5–10 years ** | 9–15 years | 9–15 years |
| Licensing pipeline |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.Y.L.; Mullally, C.A.; Haese, E.C.; Kibble, E.A.; McCluskey, N.R.; Mikucki, E.C.; Thai, V.C.; Stubbs, K.A.; Sarkar-Tyson, M.; Kahler, C.M. Anti-Virulence Therapeutic Approaches for Neisseria gonorrhoeae. Antibiotics 2021, 10, 103. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10020103
Lim KYL, Mullally CA, Haese EC, Kibble EA, McCluskey NR, Mikucki EC, Thai VC, Stubbs KA, Sarkar-Tyson M, Kahler CM. Anti-Virulence Therapeutic Approaches for Neisseria gonorrhoeae. Antibiotics. 2021; 10(2):103. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10020103
Chicago/Turabian StyleLim, Katherine Y. L., Christopher A. Mullally, Ethan C. Haese, Emily A. Kibble, Nicolie R. McCluskey, Edward C. Mikucki, Van C. Thai, Keith A. Stubbs, Mitali Sarkar-Tyson, and Charlene M. Kahler. 2021. "Anti-Virulence Therapeutic Approaches for Neisseria gonorrhoeae" Antibiotics 10, no. 2: 103. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10020103






