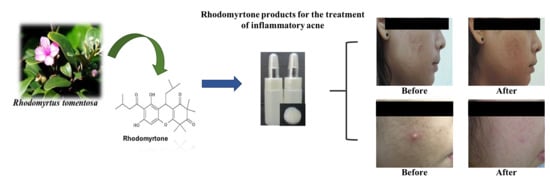
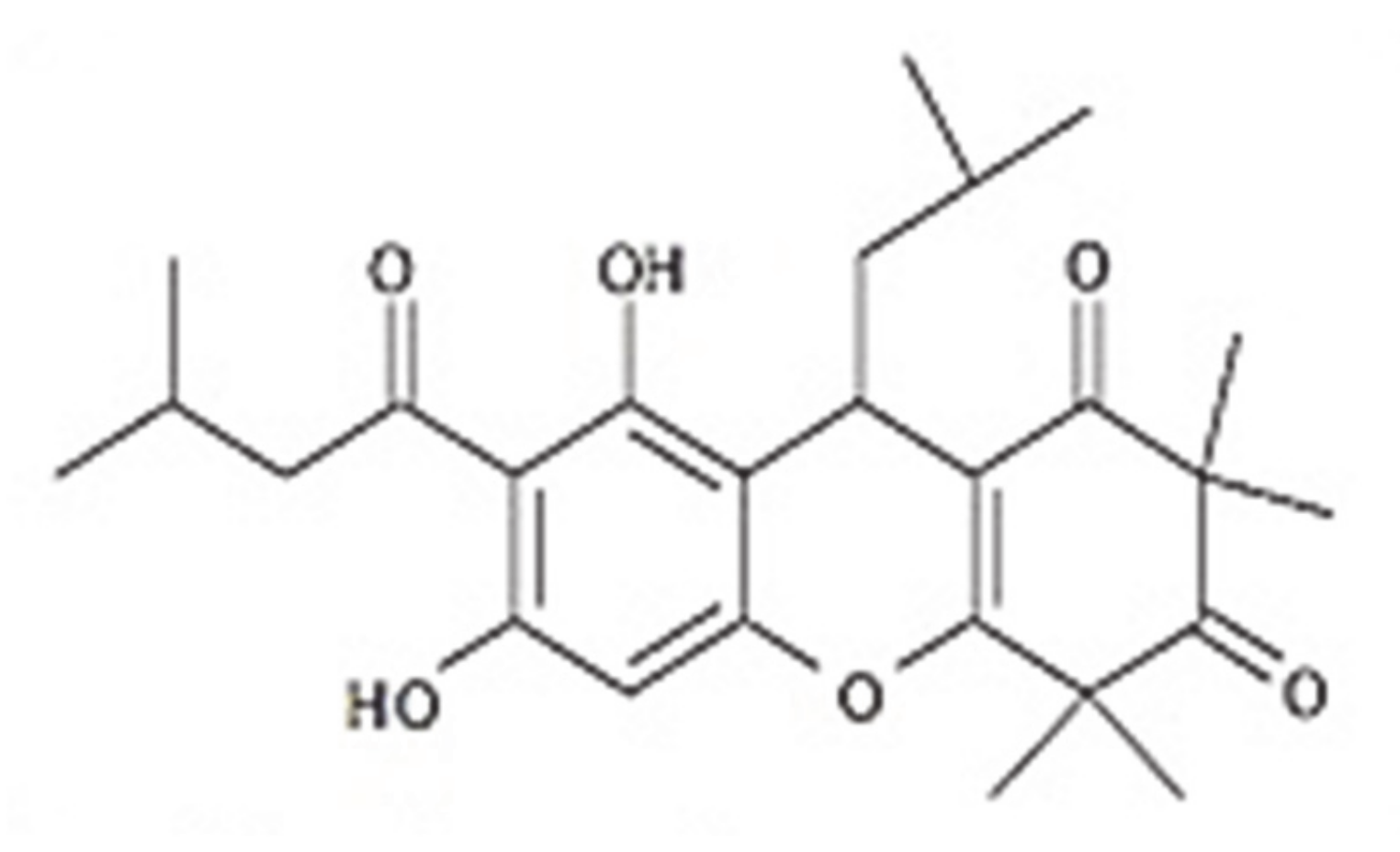
Rhodomyrtone as a New Natural Antibiotic Isolated from Rhodomyrtus tomentosa Leaf Extract: A Clinical Application in the Management of Acne Vulgaris
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Liposomal Encapsulated Rhodomyrtone Serum
3.3. Clindamycin Gel®
3.4. Clinical Study Design
3.4.1. Subject Selection
3.4.2. Efficacy Parameters
3.4.3. Safety Evaluation
3.4.4. Ethical Considerations
3.5. Customer Evaluation
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Limsuwan, S.; Trip, E.N.; Kouwen, T.R.; Piersma, S.; Hiranrat, A.; Mahabusarakam, W.; Voravuthikunchai, S.P.; van Dijl, J.M.; Kayser, O. Rhodomyrtone: A new candidate as natural antibacterial drug from Rhodomyrtus tomentosa. Phytomedicine 2009, 16, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Leejae, S.; Taylor, P.W.; Voravuthikunchai, S.P. Antibacterial mechanisms of rhodomyrtone against important hospital-acquired antibiotic-resistant pathogenic bacteria. J. Med. Microbiol. 2013, 62, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saeloh, D.; Tipmanee, V.; Jim, K.K.; Dekker, M.P.; Bitter, W.; Voravuthikunchai, S.P.; Wenzel, M.; Hamoen, L.W. The novel antibiotic rhodomyrtone traps membrane proteins in vesicles with increased fluidity. PLoS Pathog. 2018, 14, e1006876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, J.; Lukezic, T.; Kling, A.; Baumann, S.; Huttel, S.; Petkovic, H.; Müller, R. Strategies for the discovery and development of new antibiotics from natural products: Three case studies. Curr. Top. Microbiol. Immunol. 2016, 398, 339–363. [Google Scholar] [PubMed]
- Saising, J.; Voravuthikunchai, S.P. Anti Propionibacterium acnes activity of rhodomyrtone, an effective compound from Rhodomyrtus tomentosa (Aiton) Hassk. leaves. Anaerobe 2012, 18, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Chorachoo, J.; Amnuaikit, T.; Voravuthikunchai, S.P. Liposomal encapsulated rhodomyrtone: A novel antiacne drug. Evid. Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wunnoo, S.; Saising, J.; Voravuthikunchai, S.P. Rhodomyrtone inhibits lipase production, biofilm formation, and disorganizes established biofilm in Propionibacterium acnes. Anaerobe 2017, 43, 61–68. [Google Scholar] [CrossRef]
- Qidwai, A.; Pandey, M.; Pathak, S.; Kumar, R.; Dikshit, A. The emerging principles for acne biogenesis: A dermatological problem of puberty. Hum. Microbiome J. 2017, 4, 7–13. [Google Scholar] [CrossRef]
- Paithankar, D.Y.; Sakamoto, F.H.; Farinelli, W.A.; Kositratna, G.; Blomgren, R.D.; Meyer, T.J.; Faupel, L.J.; Kauvar, A.N.B.; Lloyd, J.R.; Cheung, W.L.; et al. Acne treatment based on selective photothermolysis of sebaceous follicles with topically delivered light–absorbing gold microparticles. J. Investig. Dermatol. 2015, 135, 1727–1734. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, J.; Watterson, S.; Layton, A.M.; Bjourson, A.J.; Barnard, E.; McDowell, A. Propionibacterium acnes and acne vulgaris: New insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms 2019, 7, 128. [Google Scholar] [CrossRef] [Green Version]
- Oon, H.H.; Wong, S.N.; Aw, D.C.W.; Cheong, W.K.; Goh, C.L.; Tan, H.H. Acne management guidelines by the dermatological society of Singapore. J. Clin. Aesthet. Dermatol. 2019, 12, 34–50. [Google Scholar] [PubMed]
- Biswall, I.; Gaint, R.; Kumar, N.; Mohanty, S.; Manchanda, V.; Khunger, N.; Ramesh, V.; Deb, M. In vitro antimicrobial susceptibility patterns of Propionibacterium acnes isolated from patients with acne vulgaris. J. Infect. Dev. Ctries. 2016, 10, 1140–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, B.A.; Fleischer, A.B. The extinction of topical erythromycin therapy for acne vulgaris and concern for the future of topical clindamycin. J. Dermatol. Treat. 2017, 28, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, J.S.; Spaccarelli, N.; Margolis, D.J.; James, W.D. Approaches to limit systemic antibiotic use in acne: Systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J. Am. Acad. Dermatol. 2019, 80, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Saviuc, C.; Ciubucă, B.; Dincă, G.; Bleotu, C.; Drumea, V.; Chifiriuc, M.C.; Popa, M.; Pircalabioru, G.G.; Marutescu, L.; Lazăr, V. Development and sequential analysis of a new multi-agent, anti-acne formulation based on plant-derived antimicrobial and anti-inflammatory compounds. Int. J. Mol. Sci. 2017, 18, 175. [Google Scholar] [CrossRef] [Green Version]
- Waranuch, N.; Phimnuan, P.; Yakaew, S.; Nakyai, W.; Grandmottet, F.; Onlom, C.; Srivilai, J.; Viyoch, J. Antiacne and antiblotch activities of a formulated combination of Aloe barbadensis leaf powder, Garcinia mangostana peel extract, and Camellia sinensis leaf extract. Clin. Cosmet. Investig. Dermatol. 2019, 12, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Asasutjarit, R.; Larpmahawong, P.; Fuongfuchat, A.; Sareedenchai, V.; Veeranondha, S. Physicochemical properties and anti-Propionibacterium acnes activity of film-forming solutions containing alpha-mangostin-rich extract. AAPS PharmSciTechol. 2014, 15, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Saric, S.; Notay, M.; Sivamani, K.R. Green tea and other tea polyphenols: Effects on sebum production and acne vulgaris. Antioxidants 2016, 6, 2. [Google Scholar] [CrossRef]
- Soleymani, S.; Farzaei, M.H.; Zargaran, A.; Niknam, S.; Rahimi, R. Promising plant–derived secondary metabolites for treatment of acne vulgaris: A mechanistic review. Arch. Dermatol. Res. 2020, 312, 5–23. [Google Scholar] [CrossRef]
- Archana, G.; Swati, P. Development and evaluation of Rubia cordifolia gel for treatment of acne. Res. J. Top. Cosm. Sci. 2018, 9, 39–43. [Google Scholar]
- Comin, V.M.; Lopes, L.Q.S.; Quatrin, P.M.; de Souza, M.E.; Bonez, P.C.; Pintos, F.G.; Raffin, R.P.; Vaucher, R.A.; Martinez, D.S.T.; Santos, R.C.V. Influence of Melaleuca alternifolia oil nanoparticles on aspects of Pseudomonas aeruginosa biofilm. Microb. Pathog. 2016, 93, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A. Treatment of acne with tea tree oil (melaleuca) products: A review of efficacy, tolerability and potential modes of action. Int. J. Antimicrob. Agents 2015, 45, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Ontong, J.C.; Leejae, S.; Suwalak, S.; Coote, P.J.; Voravuthikunchai, S.P. In vivo safety assessment of rhodomyrtone, a potent compound, from Rhodomyrtus tomentosa leaf extract. Toxicol. Rep. 2020, 7, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Sianglum, W.; Srimanote, P.; Taylor, P.W.; Rosado, H.; Voravuthikunchai, S.P. Transcriptome analysis of responses to rhodomyrtone in methicillin-resistant Staphylococcus aureus. PLoS ONE 2012, 7, e45744. [Google Scholar] [CrossRef] [PubMed]
- Mitsuwan, W.; Olaya-Abril, A.; Calderón-Santiago, M.; Jiménez-Munguía, I.; González-Reyes, J.A.; Priego-Capote, F.; Voravuthikunchai, S.P.; Rodríguez-Ortega, M.J. Integrated proteomic and metabolomic analysis reveals that rhodomyrtone reduces the capsule in Streptococcus pneumoniae. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Srisuwan, S.; Mackin, K.E.; Hocking, D.; Lyras, D.; Bennett-Wood, V.; Voravuthikunchai, S.P.; Robins-Browne, R.M. Antibacterial activity of rhodomyrtone on Clostridium difficile vegetative cells and spores in vitro. Int. J. Antimicrob. Agents 2018, 52, 24–729. [Google Scholar] [CrossRef] [PubMed]
- Srisuwan, S.; Voravuthikunchai, S.P. Rhodomyrtus tomentosa leaf extract inhibits methicillin-resistant Staphylococcus aureus adhesion, invasion, and intracellular survival in human HaCaT keratinocytes. Microb. Drug Resist. 2017, 23, 1002–1012. [Google Scholar] [CrossRef]
- Saising, J.; Ongsakul, M.; Voravuthikunchai, S.P. Rhodomyrtus tomentosa (Aiton) Hassk. ethanol extract and rhodomyrtone: A potential strategy for the treatment of biofilm-forming staphylococci. J. Med. Microbiol. 2011, 60, 1793–1800. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Choi, Y.M.; Lee, J.; An, I.S.; Li, L.; He, C.; Dong, Y.; Bae, S.; Meng, H. Toll-like receptor 2 plays a critical role in pathogenesis of acne vulgaris. Biomed. Dermatol. 2019, 3. [Google Scholar] [CrossRef]
- Wang, Y.; Hata, T.R.; Tong, Y.L.; Kao, M.S.; Zouboulis, C.C.; Gallo, R.L.; Huang, C.M. The anti-inflammatory activities of Propionibacterium acnes CAMP factor-targeted acne vaccines. J. Investig. Dermatol. 2018, 8, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Chorachoo, J.; Lambert, S.; Furnholm, T.; Roberts, L.; Reingold, L.; Auepemkiate, S.; Voravuthikunchai, S.P.; Johnston, A. The small molecule rhodomyrtone suppresses TNF-α and IL-17A-induced keratinocyte inflammatory responses: A potential new therapeutic for psoriasis. PLoS ONE 2018, 13, e0205340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srisuwan, S.; Tongtawe, P.; Srimanote, P.; Voravuthikunchai, S.P. Rhodomyrtone modulates innate immune responses of THP-1 monocytes to assist in clearing methicillin-resistant Staphylococcus aureus. PLoS ONE 2014, 9, e110321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hoogevest, P.; Fahir, A. Phospholipids in cosmetic carriers. In Nanocosmetics: From Ideas to Products; Cornier, J., Keck, C., Van de Voorde, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 95–140. [Google Scholar]
- Telange, D.R.; Nirgulkar, S.B.; Umekar, M.J.; Patil, A.T.; Pethe, A.M.; Bali, N.R. Enhanced transdermal permeation and anti-inflammatory potential of phospholipids complex-loaded matrix film of umbelliferone: Formulation development, physico-chemical and functional characterization. Eur. J. Pharm. Sci. 2019, 131, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Hiranrat, A.; Chitbankluoi, W.; Mahabusarakam, W.; Limsuwan, S.; Voravuthikunchai, S.P. A new flavellagic acid derivative and phloroglucinol from Rhodomyrtus tomentosa. Nat. Prod. Res. 2012, 26, 1904–1909. [Google Scholar] [CrossRef]
- Leejae, S.; Yingyongnarongkul, B.E.; Suksamrarn, A.; Voravuthikunchai, S.P. Synthesis and structure–activity relationship of rhodomyrtone derivatives as antibacterial agent. Chin. Chem. Lett. 2012, 23, 1011–1014. [Google Scholar] [CrossRef]
- US Department of Health and Human Service, Food and Drug Admistration. Center for Drug Evaluation and Research, Draft Guidance for Industry, Acne vulgaris: Developing Drugs for Treatment; Availability (draft guidance). 2005; pp. 54945–54946. Available online: https://www.federalregister.gov/documents/2005/09/19/05-18512/draft-guidance-for-industry-on-acne-vulgaris-developing-drugs-for-treatment-availability. (accessed on 4 November 2015).




| Clinical Parameter | Total (n = 60) | 1% Liposomal Encapsulated Rhodomyrtone Serum (n = 30) | 1% Clindamycin Gel (n = 30) | p-Values |
|---|---|---|---|---|
| Age (year) | 21.23 ± 2.08 a | 21.10 ± 2.02 | 21.37 ± 2.17 | 0.625 |
| Age starts having acne (year) | 15.38 ± 2.75 | 15.03 ± 1.90 | 15.73 ± 3.40 | 0.331 |
| Family with history of acne | 46.00 (76.67) b | 23.00 (76.66) | 23.00 (76.66) | 1.000 |
| Patient History of Acne Treatment | ||||
| Used to | 38.00 (63.33) | 19.00 (63.33) | 19.00 (63.33) | 1.000 |
| See doctor for acne treatment | 21.00 (35.00) | 11.00 (36.67) | 10.00 (33.33) | 0.787 |
| Use commercial drug | 27.00 (45.00) | 14.00 (46.67) | 13.00 (43.33) | 0.790 |
| Use both | 10.00 (16.67) | 6.00 (20.00) | 4.00 (13.33) | 0.488 |
| Use Facial Cleanser | ||||
| Ordinary facial cleanser | 49.00 (81.67) | 23.00 (76.67) | 26.00 (86.67) | 0.317 |
| Acne facial cleanser | 3.00 (5.00) | 1.00 (3.33) | 2.00 (6.67) | 1.000 |
| Soap | 8.00 (13.33) | 6.00 (20.00) | 2.00 (6.67) | 0.250 |
| Use Other Product | ||||
| Facial skin care | 38.00 (63.33) | 20.00 (66.67) | 18.00 (60.00) | 0.592 |
| Sunscreen | 37.00 (61.02) | 19.00 (63.33) | 18.00 (60.00) | 0.791 |
| Facial skin whitening | 1.00 (1.67) | 1.00 (3.33) | 0 | 1.000 |
| Number of Acne Lesions at Beginning | Total (n = 60) | 1% Liposomal Encapsulated Rhodomyrtone Serum (n = 30) | 1% Clindamycin Gel (n = 30) | p-Values |
|---|---|---|---|---|
| Total acne lesions | 47.02 ± 30.12 a | 45.03 ± 33.77 | 49.00 ± 26.40 | 0.614 |
| Non-inflammatory acne lesions | 38.53 ± 26.45 | 36.60 ± 29.85 | 40.47 ± 22.91 | 0.576 |
| Open comedones | 4.15 ± 6.26 | 4.03 ± 6.76 | 4.27 ± 5.83 | 0.887 |
| Closed comedones | 34.38 ± 23.20 | 32.57 ± 25.48 | 36.20 ± 20.95 | 0.549 |
| Inflammatory acne lesions | 8.48 ± 7.77 | 8.43 ± 8.48 | 8.53 ± 7.14 | 0.961 |
| Papule | 7.25 ± 6.61 | 6.67 ± 6.26 | 7.83 ± 7.01 | 0.499 |
| Pustule | 1.22 ± 2.54 | 1.73 ± 3.30 | 0.70 ± 1.29 | 0.119 |
| Nodule | 0.02 ± 0.13 | 0.03 ± 0.18 | 0 | 0.326 |
| Investigator Global Assessment (IGA) | ||||
| 1 | 5.00 (8.33) b | 1.00 (3.33) | 4.00 (3.33) | |
| 2 | 30.00 (50.00) | 18.00 (60.00) | 12.00 (40.00) | |
| 3 | 25.00 (41.67) | 11.00 (36.67) | 14.00 (36.67) | |
| 4 | 0 | 0 | 0 | |
| IGA scale | 2.33 ± 0.62 | 2.33 ± 0.55 | 2.33 ± 0.71 | 0.186 |
| Satisfaction Level | 1% Liposome Encapsulated Rhodomyrtone Serum | 1% Clindamycin Gel | Commercial Prototype |
|---|---|---|---|
| Very satisfied | 36.70 a | 40.00 a | 71.11 b |
| Moderately satisfied | 50.00 a | 43.30 a | 28.89 b |
| Slightly satisfied | 13.30 a | 16.70 a | 0 |
| Samples | IGA Scale | Numbers of Volunteers (%) | ||
|---|---|---|---|---|
| Week 2 | Week 4 | Week 8 | ||
| 1% Liposomal encapsulated rhodomyrtone serum | deteriorated (2 score) | 0 | 0 | 0 |
| deteriorated (1 score) | 3 (10.00) a | 3 (10.00) | 2 (6.67) | |
| no change | 19 (63.33) | 23 (76.67) | 21 (70.00) | |
| improved (1 score) | 8 (26.67) | 3 (10.00) | 6 (20.00) | |
| improved (2 score) | 0 | 1 (3.33) | 1 (3.33) | |
| 1% Clindamycin gel | deteriorated (2 score) | 0 | 0 | 0 |
| deteriorated (1 score) | 3 (10.00) | 4 (13.33) | 4 (13.33) | |
| no change | 22 (73.33) | 18 (60.00) | 15 (50.00) | |
| improved (1 score) | 5 (16.67) | 8 (26.67) | 9 (30.00) | |
| improved (2 score) | 0 | 0 | 2 (6.67) | |
| p-value (between groups) | 0.608 | 0.793 | 0.883 | |
| Baseline Characteristics | Rhodomyrtone Serum |
|---|---|
| Age (year) | 25.60 ± 6.11 a |
| Sex | |
| Male | 11 (24.44) b |
| Female | 34 (75.56) |
| Investigator Global Assessment (IGA) | |
| 1 | 13 (28.89) |
| 2 | 32 (71.11) |
| 3 | 0 |
| 4 | 0 |
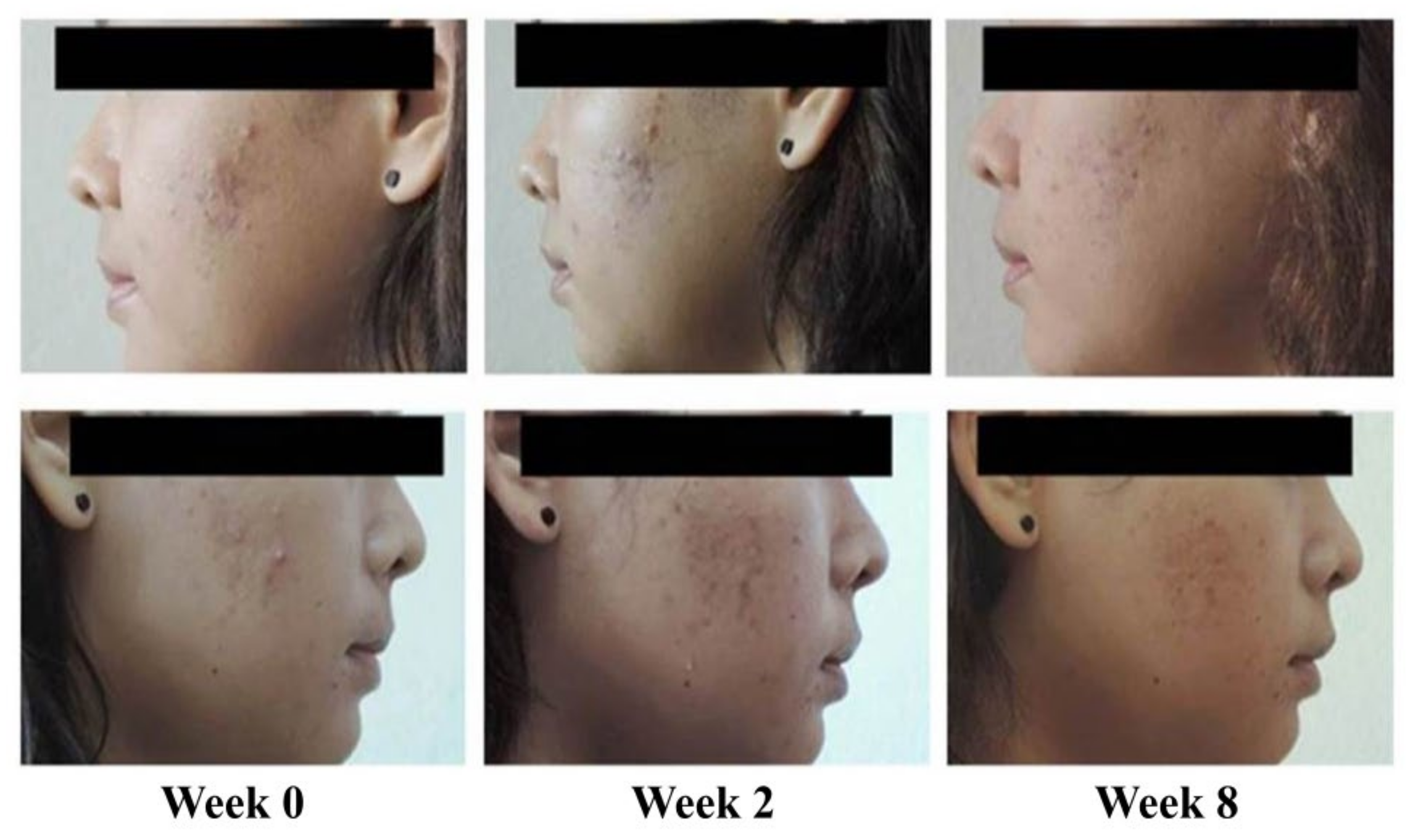
| Improvement in Inflammatory Acne | Baseline | Day 7 |
|---|---|---|
| Inflammatory Acne Lesions | ||
| Total | 3.96 ± 4.32 a | 0.89 ± 2.14 |
| Papule | 2.13 ± 3.06 | 0.58 ± 1.78 |
| Pustule | 1.82 ± 2.45 | 0.31 ± 0.82 |
| Reduction of Inflammatory Acne Lesions | ||
| Completed | 31 (68.89) b | |
| Improved | 13 (28.89) | |
| No change | 1 (2.22) | |
| IGA scale | ||
| Improved | 37 (82.22) | |
| 2 Score | 18 (40.00) | |
| 1 Score | 19 (42.22) | |
| No change | 8 (17.78) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunnoo, S.; Bilhman, S.; Amnuaikit, T.; Ontong, J.C.; Singh, S.; Auepemkiate, S.; Voravuthikunchai, S.P. Rhodomyrtone as a New Natural Antibiotic Isolated from Rhodomyrtus tomentosa Leaf Extract: A Clinical Application in the Management of Acne Vulgaris. Antibiotics 2021, 10, 108. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10020108
Wunnoo S, Bilhman S, Amnuaikit T, Ontong JC, Singh S, Auepemkiate S, Voravuthikunchai SP. Rhodomyrtone as a New Natural Antibiotic Isolated from Rhodomyrtus tomentosa Leaf Extract: A Clinical Application in the Management of Acne Vulgaris. Antibiotics. 2021; 10(2):108. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10020108
Chicago/Turabian StyleWunnoo, Suttiwan, Siwaporn Bilhman, Thanaporn Amnuaikit, Julalak C. Ontong, Sudarshan Singh, Sauvarat Auepemkiate, and Supayang P. Voravuthikunchai. 2021. "Rhodomyrtone as a New Natural Antibiotic Isolated from Rhodomyrtus tomentosa Leaf Extract: A Clinical Application in the Management of Acne Vulgaris" Antibiotics 10, no. 2: 108. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10020108