Recent Advances in Fiber–Hydrogel Composites for Wound Healing and Drug Delivery Systems
Abstract
:1. Introduction
2. Polymers Natural/Synthetic
3. Hydrogel
Hydrogel Formation: Techniques
4. Fiber
Fiber Production: Techniques
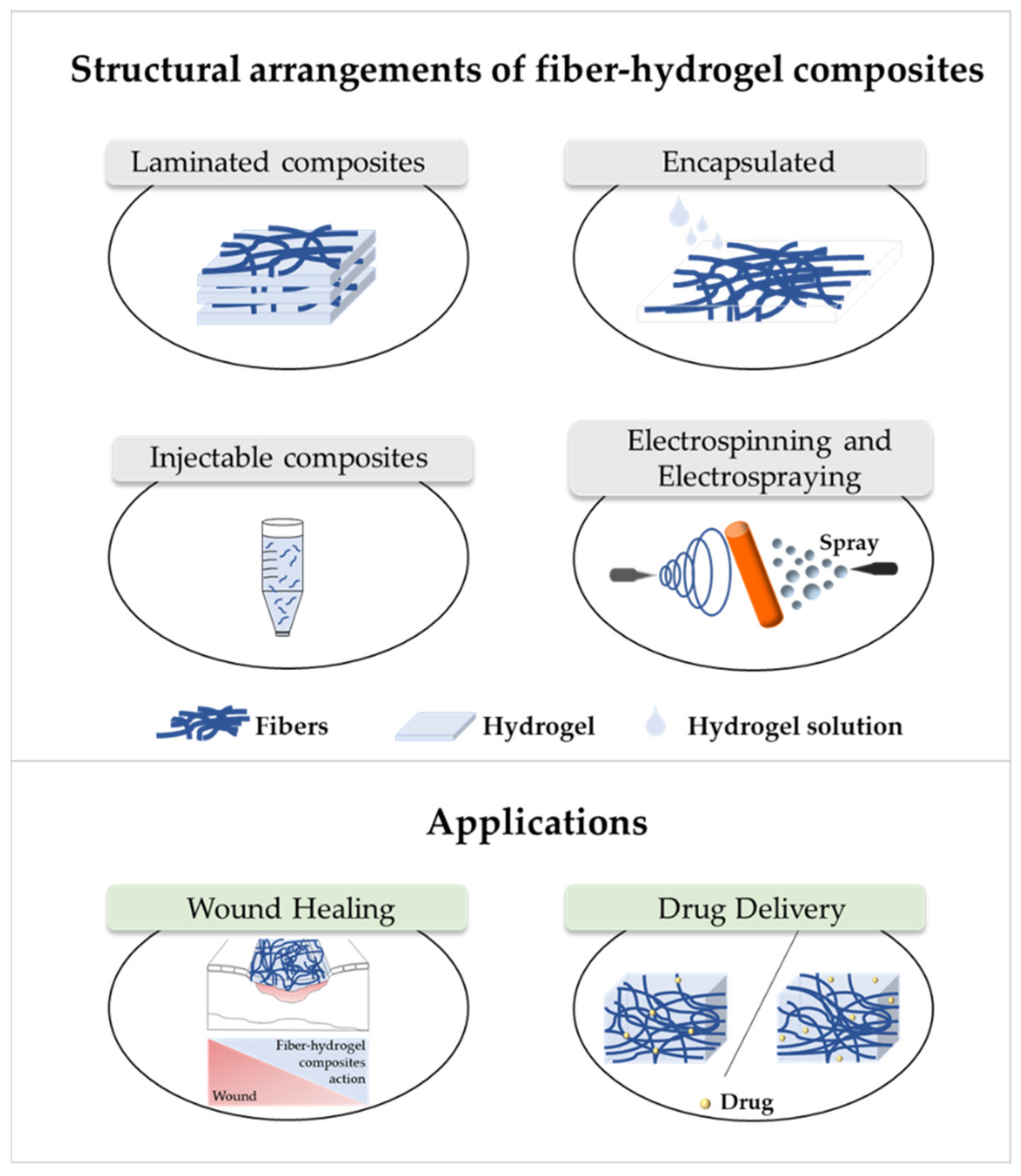
5. Fiber–Hydrogel Composites
6. Applications of Fiber–Hydrogel Composites
6.1. Wound Healing
6.2. Drug Delivery
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E.; Wagner, W.R.; Sakiyama-Elbert, S.E.; Zhang, G.; Yaszemski, M.J. Introduction to Biomaterials Science: An Evolving, Multidisciplinary Endeavor. In Biomaterials Science: An Introduction to Materials in Medicine; Wagner, W., Sakiyama-Elbert, S., Zhang, G., Yaszemski, M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 3–19. [Google Scholar]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [Green Version]
- Asim, M.; Jawaid, M.; Saba, N.; Ramengmawii; Nasir, M.; Sultan, M.T.H. Processing of hybrid polymer composites-a review. In Hybrid Polymer Composite Materials; Thakur, V.K., Thakur, M.K., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. [Google Scholar]
- Ivanova, E.P.; Bazaka, K.; Crawford, R.J. Advanced synthetic polymer biomaterials derived from organic sources. In New Functional Biomaterials for Medicine and Healthcare; Ivanova, E.P., Bazaka, K., Crawford, R.J., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 71–99. [Google Scholar]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2016, 45, 185–192. [Google Scholar] [CrossRef]
- Ribeiro, D.M.L.; Júnior, A.R.C.; de Macedo, G.H.R.V.; Chagas, V.L.; Silva, L.D.S.; da Silva Cutrim, B.; Santos, D.M.; Soares, B.L.L.; Zagmignan, A.; de Càssia Mendonça de Miranda , R.; et al. Polysaccharide-Based Formulations for Healing of Skin-Related Wound Infections: Lessons from Animal Models and Clinical Trials. Biomolecules 2020, 10, 1–16. [Google Scholar]
- Singh, M.R.; Patel, S.; Singh, D. Natural polymer-based hydrogels as scaffolds for tissue engineering. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 231–260. [Google Scholar]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of natural polymers for advanced wound care: Towards responsive and adaptive dressings. J. Mater. Chem. B 2016, 4, 4801–4812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bealer, E.J.; Onissema-Karimu, S.; Rivera-Galletti, A.; Francis, M.; Wilkowski, J.; la Cruz, D.S.; Hu, X. Protein–Polysaccharide Composite Materials: Fabrication and Applications. Polymer 2020, 12, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, C.S.; Ribeiro, A.R.M.; Homem, N.C.; Felgueiras, H.P. Spun Biotextiles in Tissue Engineering and Biomolecules Delivery Systems. Antibiotics 2020, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Vandghanooni, S.; Eskandani, M. Natural polypeptides-based electrically conductive biomaterials for tissue engineering. Int. J. Biol. Macromol. 2020, 147, 706–733. [Google Scholar] [CrossRef] [PubMed]
- Poole-Warren, L.A.; Patton, A.J. Introduction to biomedical polymers and biocompatibility. In Biosynthetic Polymers for Medical Applications; Poole-Warren, L., Martens, P., Green, R., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 3–31. [Google Scholar]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Mondal, D.; Mollick, M.M.R.; Bhowmick, B.; Maity, D.; Bain, M.K.; Rana, D.; Mukhopadhyay, A.; Dana, K.; Chattopadhyay, D. Effect of poly(vinyl pyrrolidone) on the morphology and physical properties of poly(vinyl alcohol)/sodium montmorillonite nanocomposite films. Prog. Nat. Sci. Mater. Int. 2013, 23, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Salehi-Nik, N.; Rezai Rad, M.; Nazeman, P.; Khojasteh, A. Polymers for oral and dental tissue engineering. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 25–46. [Google Scholar]
- Duskey, J.T.; Baraldi, C.; Gamberini, M.C.; Ottonelli, I.; Da Ros, F.; Tosi, G.; Forni, F.; Vandelli, M.A.; Ruozi, B. Investigating Novel Syntheses of a Series of Unique Hybrid PLGA-Chitosan Polymers for Potential Therapeutic Delivery Applications. Polymers 2020, 12, 823. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [Green Version]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.D.N.; Park, K.D.; Ching, Y.C.; Huynh, C.; Nguyen, D.H. A Comprehensive Review on Polymeric Hydrogel and Its Composite: Matrices of Choice for Bone and Cartilage Tissue Engineering. J. Ind. Eng. Chem. 2020, 89, 58–82. [Google Scholar] [CrossRef]
- Felgueiras, H.P.P.; Wang, L.-M.; Ren, K.-F.; Querido, M.M.; Jin, Q.; Barbosa, M.; Ji, J.; Martins, M.C.L. Octadecyl chains immobilized onto hyaluronic acid coatings by thiolene “click chemistry“ increase the surface antimicrobial properties and prevent platelet adhesion and activation to polyurethane. ACS Appl. Mater. Interfaces 2017, 9, 7979–7989. [Google Scholar] [CrossRef]
- Shi, W.; Hass, B.; Kuss, M.A.; Zhang, H.; Ryu, S.; Zhang, D.; Li, T.; Li, Y.; Duan, B. Fabrication of versatile dynamic hyaluronic acid-based hydrogels. Carbohydr. Polym. 2020, 233, 115803. [Google Scholar] [CrossRef]
- Dave, P.N.; Gor, A. Natural Polysaccharide-Based Hydrogels and Nanomaterials: Recent Trends and Their Applications. In Handbook of Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 36–66. [Google Scholar]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef]
- Antunes, J.C.; Conçalves, R.M.; Barbosa, M.A. Chitosan/Poly(γ-glutamic acid) Polyelectrolyte Complexes: From Self-Assembly to Application in Biomolecules Delivery and Regenerative Medicine. Res. Rev. J. Mater. Sci. 2016, 4, 12–36. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical and applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezvanain, M.; Ahmad, N.; Amin, M.C.I.M.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Chen, B.; Zeng, X.; Sun, Y.; Tang, X.; Lin, L. Recent advances on sustainable cellulosic materials for pharmaceutical carrier applications. Carbohydr. Polym. 2020, 244, 116492. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods–A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.A.; Paiva, M.C.; Amorim, M.T.P.; Felgueiras, H.P. Electrospun Nanocomposites Containing Cellulose and Its Derivatives Modified with Specialized Biomolecules for an Enhanced Wound Healing. Nanomaterials 2020, 10, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.-H.; Qi, C.; Ma, M.-G.; Wan, P. Multifunctional cellulose-based hydrogels for biomedical applications. J. Mater. Chem. B 2019, 7, 1541–1562. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.-M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, A.W.-M.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage Tissue Engineering Application of Injectable Gelatin Hydrogel with In Situ Visible-Light-Activated Gelation Capability in both Air and Aqueous Solution. Tissue Eng. Part A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Lin, H.; Rothrauff, B.B.; Yu, S.; Tuan, R.S. Multilayered Polycaprolactone/Gelatin Fiber-Hydrogel Composite for Tendon Tissue Engineering. Acta Biomater. 2016, 35, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurumurthy, B.; Janorkar, A.V. Improvements in Mechanical Properties of Collagen-Based Scaffolds for Tissue Engineering. Curr. Opin. Biomed. Eng. 2021, 17, 100253. [Google Scholar] [CrossRef]
- Nuñez, S.M.; Guzmán, F.; Valencia, P.; Almonacid, S.; Cárdenas, C. Collagen as a source of bioactive peptides: A bioinformatics approach. Electron. J. Biotechnol. 2020, 48, 101–108. [Google Scholar] [CrossRef]
- Hashim, P.; Mohd Ridzwan, M.S.; Bakar, J.; Mat Hashim, D. Collagen in food and beverage industries. Int. Food Res. J. 2015, 22, 1–8. [Google Scholar]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Yu, F.; Prashantha, K.; Soulestin, J.; Lacrampe, M.-F.; Krawczak, P. Plasticized-starch/poly(ethylene oxide) blends prepared by extrusion. Carbohydr. Polym. 2013, 91, 253–261. [Google Scholar] [CrossRef]
- Theodosopoulos, G.V.; Zisis, C.; Charalambidis, G.; Nikolaou, V.; Coutsolelos, A.G.; Pitsikalis, M. Synthesis, Characterization and Thermal Properties of Poly(ethylene oxide), PEO, Polymacromonomers via Anionic and Ring Opening Metathesis Polymerization. Polymers 2017, 9, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, J.; Pati, F.; Jeong, Y.H.; Cho, D.-W. Biomaterials for Biofabrication of 3D Tissue Scaffolds. In Biofabrication; Forgacs, G., Sun, W., Eds.; William Andrew Publishing: Norwich, NY, USA, 2013; pp. 23–46. [Google Scholar]
- Wong, R.S.H.; Dodou, K. Effect of Drug Loading Method and Drug Physicochemical Properties on the Material and Drug Release Properties of Poly (Ethylene Oxide) Hydrogels for Transdermal Delivery. Polymer 2017, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Ramakrishna, S. Biocompatible nanofiber matrices for the engineering of a dermal substitute for skin regeneration. Tissue Eng. 2005, 11, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Ji, C.; Chan, A.K.L.; Kazarian, S.G.; Ruys, A.; Dehghani, F. Fabrication of chitosan/poly(ε-caprolactone) composite hydrogels for tissue engineering applications. J. Mater. Sci. Mater. Med. 2011, 22, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 7. [Google Scholar] [CrossRef] [Green Version]
- Manavitehrani, I.; Fathi, A.; Wang, Y.; Maitz, P.; Dehghani, F. Reinforced Poly(Propylene Carbonate) Composite with Enhanced and Tunable Characteristics, an Alternative for Poly(lactic Acid). ACS Appl. Mater. Interfaces 2015, 7, 22421–22430. [Google Scholar] [CrossRef] [PubMed]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurface Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef] [Green Version]
- Virlan, M.J.R.; Miricescu, D.; Totan, A.; Greabu, M.; Tanase, C.; Sabliov, C.M.; Caruntu, C.; Calenic, B. Current Uses of Poly(lactic-co-glycolic acid) in the Dental Field: A Comprehensive Review. J. Chem. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly(Lactic-co-Glycolic Acid): Applications and Future Prospects for Periodontal Tissue Regeneration. Polymer 2017, 9, 189. [Google Scholar] [CrossRef]
- Del Prado, A.; Civantos, A.; Martínez-Campos, E.; Levkin, P.A.; Reinecke, H.; Gallardo, A.; Elvira, C. Efficient and Low Cytotoxicity Gene Carriers Based on Amine-Functionalized Polyvinylpyrrolidone. Polymers 2020, 12, 2724. [Google Scholar] [CrossRef]
- Voronova, M.; Rubleva, N.; Kochkina, N.; Afineevskii, A.; Zakharov, A.; Surov, O. Preparation and Characterization of Polyvinylpyrrolidone/Cellulose Nanocrystals Composites. Nanomaterials 2018, 8, 1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurakula, M.; Rao, G.K. Moving polyvinyl pyrrolidone electrospun nanofibers and bioprinted scaffolds toward multidisciplinary biomedical applications. Eur. Polym. J. 2020, 136, 109919. [Google Scholar] [CrossRef]
- Mc Gann, M.J.; Higginbotham, C.L.; Geever, L.M.; Nugent, M.J.D. The synthesis of novel pH-sensitive poly(vinyl alcohol) composite hydrogels using a freeze/thaw process for biomedical applications. Int. J. Pharm. 2009, 372, 154–161. [Google Scholar] [CrossRef]
- Costa-Júnior, E.S.; Barbosa-Stancioli, E.F.; Mansur, A.A.P.; Vasconcelos, W.L.; Mansur, H.S. Preparation and characterization of chitosan/poly(vinyl alcohol) chemically crosslinked blends for biomedical applications. Carbohydr. Polym. 2009, 76, 472–481. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [Green Version]
- Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, I.Y.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S.; et al. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS PharmSciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Suo, H.; Zhang, D.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C 2018, 92, 612–620. [Google Scholar] [CrossRef]
- Hernández-González, A.C.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Alginate hydrogels for bone tissue engineering, from injectables to bioprinting: A Review. Carbohydr. Polym. 2019, 229, 115514. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L.; Zhao, C.; Ding, J.; Chen, X. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsou, Y.-H.; Khoneisser, J.; Huang, P.-C.; Xu, X. Hydrogel as a bioactive material to regulate stem cell fate. Bioact. Mater. 2016, 1, 39–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasian, M.; Massoumi, B.; Mohammad-Rezaei, R.; Samadian, H.; Jaymand, M. Scaffolding polymeric biomaterials: Are naturally occurring biological macromolecules more appropriate for tissue engineering? Int. J. Biol. Macromol. 2019, 134, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [Green Version]
- White, J.C.; Saffe, E.M.; Bhatia, S.R. Alginate/PEO-PPO-PEO Composite Hydrogels with Thermally-Active Plasticity. Biomacromolecules 2013, 14, 4456–4464. [Google Scholar] [CrossRef]
- Bai, Z.; Wang, T.; Zheng, X.; Huang, Y.; Chen, Y.; Dan, W. High strength and bioactivity polyvinyl alcohol/collagen composite hydrogel with tannic acid as cross-linker. Polym. Eng. Sci. 2020, 1–10. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Burkert, S.; Schmidt, T.; Gohs, U.; Dorschner, H.; Arndt, K.-F. Cross-linking of poly (N-vinyl pyrrolidone) films by electron beam irradiation. Radiat. Phys. Chem. 2007, 76, 1324–1328. [Google Scholar] [CrossRef]
- Yang, L.; Chu, J.S.; Fix, J.A. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002, 235, 1–15. [Google Scholar] [CrossRef]
- Teijón, C.; Guerrero, S.; Olmo, R.; Teijón, J.M.; Blanco, M.D. Swelling Properties of Copolymeric Hydrogels of Poly(ethylene glycol) Monomethacrylate and Monoesters of Itaconic Acid for Use in Drug Delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 716–726. [Google Scholar] [CrossRef]
- Lowman, A.M.; Peppas, N.A. Analysis of the Complexation/Decomplexation Phenomena in Graft Copolymer Networks. Macromolecular 1997, 30, 4959–4965. [Google Scholar] [CrossRef]
- Jana, S.; Saha, A.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Aceclofenac-loaded chitosan-tamarind seed polysaccharide interpenetrating polymeric network microparticles. Colloids Surf. B Biointerfaces 2013, 105, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Okay, O. Semicrystalline physical hydrogels with shape-memory and self-healing properties. J. Mater. Chem. B 2019, 7, 1581–1596. [Google Scholar] [CrossRef]
- BaoLin, G.; MA, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar]
- Iglesias, N.; Galbis, E.; Valencia, C.; Díaz-Blanco, M.J.; Lacroix, B.; De-Paz, M.-V. Biodegradable double cross-linked chitosan hydrogels for drug delivery: Impact of chemistry on rheological and pharmacological performance. Int. J. Biol. Macromol. 2020, 165, 2205–2218. [Google Scholar] [CrossRef]
- Kong, H.J.; Kaigler, D.; Kim, K.; Mooney, D.J. Controlling Rigidity and Degradation of Alginate Hydrogels via Molecular Weight Distribution. Biomacromolecules 2004, 5, 1720–1727. [Google Scholar] [CrossRef]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- West, J.L.; Hubbell, J.A. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules 1999, 32, 241–244. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel Biodegradable Hydrogel Based on Natural Polymers: Synthesis, Characterization, Swelling/Reswelling and Biodegradability. Eur. Polym. J. 2018, 112, 678–687. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fänger, C.; Wack, H.; Ulbricht, M. Macroporous Poly(N-isopropylacrylamide) Hydrogels with Adjustable size “Cut-off” for the Efficient and Reversible Immobilization of Biomacromolecules. Macromol. Biosci. 2006, 6, 393–402. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2014, 7544, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bag, M.A.; Valenzuela, L.M. Impact of the Hydration States of Polymers on Their Hemocompatibility for Medical Applications: A review. Int. J. Mol. Sci. 2017, 18, 1422. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical properties of pH-sensitive hydrogels based on hydroxyethyl cellulose–hyaluronic acid and for applications as transdermal delivery systems for skin lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Salleh, K.M.; Zakaria, S.; Sajab, M.S.; Gan, S.; Chia, C.H.; Jaafar, S.N.S.; Amran, U.A. Chemically crosslinked hydrogel and its driving force towards superabsorbent behaviour. Int. J. Biol. Macromol. 2018, 118, 1422–1430. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Ji, Q.X.; Xing, K.; Li, X.Y.; Liu, C.S.; Chen, X.G. Preparation and characteristics of novel porous hydrogel films based on chitosan and glycerophosphate. Carbohydr. Polym. 2009, 76, 410–416. [Google Scholar] [CrossRef]
- Kimura, M.; Fukumoto, K.; Watanabe, J.; Ishihara, K. Hydrogen-bonding-driven spontaneous gelation of water-soluble phospholipid polymers in aqueous medium. J. Biomater. Sci. Polym. Ed. 2004, 15, 631–644. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and Self-healing Hydrogels Formed via Hydrophobic Interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Stenekes, R.J.H.; Talsma, H.; Hennink, W.E. Formation of dextran hydrogels by crystallization. Biomaterials 2001, 22, 1891–1898. [Google Scholar] [CrossRef]
- George, J.; Hsu, C.-C.; Nguyen, L.T.B.; Ye, H.; Cui, Z. Neural tissue engineering with structured hydrogels in CNS models and therapies. Biotechnol. Adv. 2019, 42, 1–17. [Google Scholar] [CrossRef]
- Czarnecki, S.; Rossow, T.; Seiffert, S. Hybrid Polymer-Network Hydrogels with Tunable Mechanical Response. Polymers 2016, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002, 43, 3–12. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze-thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, Y.-T.; Xie, X.-M. Self-healable, super tough graphene oxide/poly(acrylic acid) nanocomposite hydrogels facilitated by dual cross-linking effects through dynamic ionic interactions. J. Mater. Chem. B 2015, 3, 4001–4008. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhanga, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymers 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Abdurrahmanoglu, S.; Can, V.; Okay, O. Design of high-toughness polyacrylamide hydrogels by hydrophobic modification. Polymer 2009, 50, 5449–5455. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Yang, W.; Jiang, W.; Chen, F.; Fu, Q. Enhancement of mechanical property and absorption capability of hydrophobically associated polyacrylamide hydrogels by adding cellulose nanofiber. Mater. Res. Express 2020, 7, 015319. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Vélaz, I.; Peñas, F.J.; Zavala-Rivera, P.; Rosas-Durazo, A.J.; Maldonado-Arce, A.D.; Martínez-Barbosa, M.E. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. X-ray Diffraction Analysis of Poly (vinyl alcohol) Hydrogels, Obtained by Freezing and Thawing Techniques. Macromolecules 2004, 37, 1921–1927. [Google Scholar] [CrossRef]
- Lotfipour, F.; Alami-Milani, M.; Salatin, S.; Hadavi, A.; Jelvehgari, M. Freeze-thaw- induced cross-linked PVA/chitosan for oxytetracycline-loaded wound dressing: The experimental design and optimization. Res. Pharm. Sci. 2019, 14, 175–189. [Google Scholar]
- Sabnis, A.; Rahimi, M.; Chapman, C.; Nguyen, K.T. Cytocompatibility studies of an in situ photopolymerized thermoresponsive hydrogel nanoparticle system using human aortic smooth muscle cells. J. Biomed. Mater. Res. Part A 2008, 91, 52–59. [Google Scholar]
- Han, W.T.; Jang, T.; Chen, S.; Chong, L.S.H.; Jung, H.; Song, J. Improved cell viability for large-scale biofabrication with photo-crosslinkable hydrogel systems through a dual-photoinitiator approach. Biomater. Sci. 2020, 8, 450–461. [Google Scholar] [CrossRef]
- Zhao, L.; Li, X.; Zhao, J.; Ma, S.; Ma, X.; Fan, D.; Zhu, C.; Liu, Y. A novel smart injectable hydrogel prepared by microbial transglutaminase and human-like collagen: Its characterization and biocompatibility. Mater. Sci. Eng. C 2016, 68, 317–326. [Google Scholar] [CrossRef]
- Ren, K.; He, C.; Cheng, Y.; Li, G.; Chen, X. Injectable enzymatically crosslinked hydrogels based on a poly(L-glutamic acid) graft copolymer. Polym. Chem. 2014, 5, 5069–5076. [Google Scholar] [CrossRef]
- Teixeira, L.S.M.; Feijen, J.; Van Blitterswijk, C.A.; Dijkstra, P.J.; Karperien, M. Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Kanafi, N.M.; Rahman, N.A.; Rosdi, N.H. Citric acid cross-linking of highly porous carboxymethyl cellulose poly(ethylene oxide) composite hydrogel films for controlled release applications. Mater. Today Proc. 2019, 7, 721–731. [Google Scholar] [CrossRef]
- Dimida, S.; Demitri, C.; De Benedictis, V.M.; Scalera, F.; Gervaso, F.; Sannino, A. Genipin-cross-linked chitosan-based hydrogels: Reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Lai, J.-Y. Solvent Composition is Critical for Carbodiimide Cross-Linking of Hyaluronic Acid as an Ophthalmic Biomaterial. Materials 2012, 5, 1986–2002. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Teixeira, L.S.M.; Krouwels, A.; Dijkstra, P.J.; Van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010, 6, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-Linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, M.; Rodriguez-Berna, G.; Gonzalez-Alvarez, I.; Hernández, M.J.; Corma, A.; Bermejo, M.; Merino, V.; Gonzalez-Alvarez, M. Ionic hydrogel based on chitosan crosslinked with 6-Phosphogluconic Trisodium salt as a drug delivery system. Biomacromolecules 2018, 19, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Choi, O.K.; Lee, J.; Noh, J.; Lee, S.; Park, A.; Rim, M.A.; Reis, R.L.; Khang, G. Evaluation of double network hydrogel of poloxamer-heparin/gellan gum for bone marrow stem cells delivery carrier. Colloids Surfaces B Biointerfaces 2019, 181, 879–889. [Google Scholar] [CrossRef]
- Masruchin, N.; Park, B.-D.; Causin, V. Influence of sonication treatment on supramolecular cellulose microfibril-based hydrogels induced by ionic interaction. J. Ind. Eng. Chem. 2015, 29, 265–272. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.-N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly(vinyl alcohol)/poly(acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6, 8105–8114. [Google Scholar] [CrossRef]
- Ye, X.; Li, X.; Shen, Y.; Chang, G.; Yang, J.; Gu, Z. Self-healing pH-sensitive cytosine- and guanosine-modified hyaluronic acid hydrogels via hydrogen bonding. Polymer 2017, 108, 348–360. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Mandal, U.K.; Taher, M.; Susanti, D.; Jaffri, J.M. PVA-PEG physically cross-linked hydrogel film as a wound dressing: Experimental design and optimization. Pharm. Dev. Technol. 2018, 23, 751–760. [Google Scholar] [CrossRef]
- Hurtado, M.M.; de Vries, E.G.; Zeng, X.; van der Heide, E. A tribo-mechanical analysis of PVA-based building-blocks for implementation in a 2-layered skin model. J. Mech. Behav. Biomed. Mater. 2016, 62, 319–332. [Google Scholar] [CrossRef]
- Nachlas, A.L.Y.; Li, S.; Jha, R.; Singh, M.; Xu, C.; Davis, M.E. Human iPSC-derived mesenchymal stem cells matured into valve interstitial- like cells using PEGDA hydrogels. Acta Biomater. 2018, 71, 235–246. [Google Scholar] [CrossRef]
- Yoon, H.J.; Shin, S.R.; Cha, J.M.; Lee, S.H.; Kim, J.H.; Do, J.T.; Song, H.; Bae, H. Cold Water Fish Gelatin Methacryloyl Hydrogel for Tissue Engineering Application. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef]
- Besser, R.R.; Bowles, A.C.; Alassaf, A.; Carbonero, D.; Claure, I.; Jones, E.; Reda, J.; Wubker, L.; Batchelor, W.; Ziebarth, N.; et al. Enzymatically crosslinked gelatin-laminin hydrogels for applications in neuromuscular tissue engineering. Biomater. Sci. 2020, 8, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, M.; Murtaza, G. Fabrication and characterization of genipin cross-linked chitosan/gelatin hydrogel for pH-sensitive, oral delivery of metformin with an application of response surface methodology. Int. J. Biol. Macromol. 2018, 114, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mejía, G.; Vázquez-Torres, N.A.; Castell-Rodríguez, A.; del Río, J.M.; Corea, M.; Jiménez-Juárez, R. Synthesis of new chitosan-glutaraldehyde scaffolds for tissue engineering using schiff reactions. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 579, 123658. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Huang, L. Feasibility of chitosan-alginate (Chi-Alg) hydrogel used as scaffold for neural tissue engineering: A pilot study in vitro. Biotechnol. Biotechnol. Equip. 2017, 31, 766–773. [Google Scholar] [CrossRef] [Green Version]
- Seera, S.D.K.; Kundu, D.; Banerjee, T. Physical and chemical crosslinked microcrystalline cellulose-polyvinyl alcohol hydrogel: Freeze–thaw mediated synthesis, characterization and in vitro delivery of 5- fluorouracil. Cellulose 2020, 27, 6521–6535. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. Part A 2020, 108, 1617–1633. [Google Scholar] [CrossRef]
- Moulherat, C.; Tengberg, M.; Haquet, J.F.; Mille, B. First evidence of cotton at Neolithic Mehrgarh, Pakistan: Analysis of Mineralized Fibres from a Copper Bead. J. Archaeol. Sci. 2002, 29, 1393–1401. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2018, 8, 1800465. [Google Scholar] [CrossRef] [Green Version]
- Bari, E.; Morrell, J.J.; Sistani, A. Durability of natural/synthetic/biomass fiber-based polymeric composites: Laboratory and field tests. In Durability and Life Prediction in Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Jawaid, M., Thariq, M., Saba, N., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 15–26. [Google Scholar]
- Tavares, T.D.; Antunes, J.C.; Ferreira, F.; Felgueiras, H.P. Biofunctionalization of natural Fiber-Reinforced Biocomposites for Biomedical Applications. Biomolecules 2020, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The use of electrospun curcumin-loaded poly(L-lactic acid) fiber mats as wound dressing materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Heydari, Z.; Mohebbi-Kalhori, D.; Afarani, M.S. Engineered electrospun polycaprolactone (PCL)/octacalcium phosphate (OCP) scaffold for bone tissue engineering. Mater. Sci. Eng. C 2017, 81, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Windbergs, M. Controlled dual drug release by coaxial electrospun fibers–Impact of the core fluid on drug encapsulation and release. Int. J. Pharm. 2018, 556, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. A review of the Recent Developments in Biocomposites Based on Natural Fibres and Their Application Perspectives. Compos. Part A Appl. Sci. Manuf. 2015, 77, 1–25. [Google Scholar] [CrossRef]
- Hao, L.C.; Sapuan, S.M.; Hassan, M.R.; Sheltami, R.M. Natural fiber reinforced vinyl polymer composites. In Natural Fibre Reinforced Vinyl Ester and Vinyl Polymer Composites; Sapuan, S.M., Ismail, H., Zainudin, E.S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 27–70. [Google Scholar]
- Balla, V.K.; Kate, K.H.; Satyavolu, J.; Singh, P.; Tadimeti, J.G.D. Additive manufacturing of natural fiber reinforced polymer composites: Processing and prospects. Compos. Part B Eng. 2019, 174, 106956. [Google Scholar] [CrossRef]
- Fidelis, M.E.A.; Pereira, T.V.C.; Gomes, O.D.F.M.; de Andrade Silva, F.; Filho, R.D.T. The effect of fiber morphology on the tensile strength of natural fibers. Integr. Med. Res. 2013, 2, 149–157. [Google Scholar]
- Rahman, R.; Putra, S.Z.F.S. Tensile properties of natural and synthetic fiber-reinforced polymer composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Jawaid, M., Thariq, M., Saba, N., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 81–102. [Google Scholar]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and Properties of Natural Fiber Polymer Composites: A Comprehensive Review. J. Clean. Prod. 2017, 172, 566–581. [Google Scholar] [CrossRef]
- Bannow, J.; Benjamins, J.-W.; Wohlert, J.; Löbmann, K.; Svagan, A.J. Solid nanofoams based on cellulose nanofibers and indomethacin–the effect of processing parameters and drug content on material structure. Int. J. Pharm. 2017, 526, 291–299. [Google Scholar] [CrossRef]
- Doench, I.; Torres-Ramos, M.E.W.; Montembault, A.; de Oliveira, P.N.; Halimi, C.; Viguier, E.; Heux, L.; Siadous, R.; Thiré, R.M.S.M.; Osorio-Madrazo, A. Injectable and Gellable Chitosan Formulations Filled with Cellulose Nanofibers for Intervertebral Disc Tissue Engineering. Polymers 2018, 10, 1202. [Google Scholar] [CrossRef] [Green Version]
- Ramamoorthy, S.K.; Skrifvars, M.; Persson, A. A Review of Natural Fibers Used in Biocomposites: Plant, Animal and Regenerated Cellulose Fibers. Polym. Rev. 2015, 55, 107–161. [Google Scholar] [CrossRef]
- Costa, F.; Silva, R.; Boccaccini, A.R. Fibrous protein-based biomaterials (silk, keratin, elastin, and resilin proteins) for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 175–204. [Google Scholar]
- McLellan, J.; Thornhill, S.G.; Shelton, S.; Kumar, M. Keratin-Based Biofilms, Hydrogels, and Biofibers. In Keratin as a Protein Biopolymer; Sharma, S., Kumar, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 187–200. [Google Scholar]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Babu, K.M. Natural Textile Fibres: Animal and Silk Fibres. In Textiles and Fashion; Sinclair, R., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 57–78. [Google Scholar]
- Mahltig, B. Introduction to inorganic fibers. In Inorganic and Composite Fibers: Production, Properties, and Applications; Mahltig, B., Kyosev, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–29. [Google Scholar]
- Bhat, G.; Kandagor, V. Synthetic polymer fibers and their processing requirements. In Advances in Filament Yarn Spinning of Textiles and Polymers; Zhang, D., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 3–30. [Google Scholar]
- Ivorra-Martinez, J.; Verdu, I.; Fenollar, O.; Sanchez-Nacher, L.; Balart, R.; Quiles-Carrillo, L. Manufacturing and Properties of Binary Blend from Bacterial Polyester Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and Poly(caprolactone) with Improved Toughness. Polymers 2020, 12, 1118. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- McNeil, S.E.; Griffiths, H.R.; Perrie, Y. Polycaprolactone Fibres as a Potential Delivery System for Collagen to Support Bone Regeneration. Curr. Drug Deliv. 2011, 8, 448–455. [Google Scholar] [CrossRef]
- Hu, W.-W.; Lin, C.-H.; Hong, Z.-J. The enrichment of cancer stem cells using composite alginate/polycaprolactone nanofibers. Carbohydr. Polym. 2018, 206, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Levengood, S.L.; Erickson, A.E.; Chang, F.; Zhang, M. Chitosan-poly(caprolactone) nanofibers for skin repair. J. Mater. Chem. B 2017, 5, 1822–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kan, Y.; Salimon, A.I.; Korsunsky, A.M. On the electrospinning of nanostructured collagen-PVA fiber mats. Mater. Today Proc. 2020, 33, 2013–2019. [Google Scholar] [CrossRef]
- Naskar, D.; Ghosh, A.K.; Mandal, M.; Das, P.; Nandi, S.K.; Kundu, S.C. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials 2017, 136, 67–85. [Google Scholar] [CrossRef]
- Latif, R.; Wakeel, S.; Khan, N.Z.; Siddiquee, A.N.; Verma, S.L.; Khan, Z.A. Surface treatments of plant fibers and their effects on mechanical properties of fiber-reinforced composites: A review. J. Reinf. Plast. Compos. 2018, 38, 15–30. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Arpitha, G.R.; Naik, L.L.; Gopalakrisha, K.; Yogesha, B. Applications of Natural Fibers and Its Composites: An Overview. Nat. Resour. 2016, 7, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Peças, P.; Carvalho, H.; Salman, H.; Leite, M. Natural Fibre Composites and Their Applications: A Review. J. Compos. Sci. 2018, 2, 66. [Google Scholar] [CrossRef] [Green Version]
- Padil, V.V.T.; Cheong, J.Y.; KP, A.; Makvandi, P.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Černík, M.; Kim, I.D.; Varma, R.S. Electrospun fibers based on carbohydrate gum polymers and their multifaceted applications. Carbohydr. Polym. 2020, 247, 116705. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surfaces B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef]
- Shabani, I.; Haddadi-Asl, V.; Seyedjafari, E.; Soleimani, M. Cellular infiltration on nanofibrous scaffolds using a modified electrospinning technique. Biochem. Biophys. Res. Commun. 2012, 423, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Kumbar, S.G.; James, R.; Nukavarapu, S.P.; Laurencin, C.T. Electrospun nanofiber scaffolds: Engineering soft tissues. Biomed. Mater. 2008, 3, 034002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hazeem, N.Z.A. Nanofibers and Electrospinning Method. In Novel Nanomaterials-Synthesis and Applications. Kyzas, G., Mitropoulos, A.C., Eds.; IntechOpen: London, UK, 2018; pp. 191–210. [Google Scholar]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chemie Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F. Wet-spinning of Biomedical Polymers: From Single Fibers Production to Additive Manufacturing of 3D Scaffolds. Polym. Int. 2017, 66, 1690–1696. [Google Scholar] [CrossRef]
- Ozipek, B.; Karakas, H. Wet spinning of synthetic polymer fibers. In Advances in Filament Yarn Spinning of Textiles and Polymers; Zhang, D., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 174–186. [Google Scholar]
- Tronci, G.; Kanuparti, R.S.; Arafat, M.T.; Yin, J.; Wood, D.J.; Russell, S.J. Wet-spinnability and crosslinked fibre properties of two collagen polypeptides with varied molecular weight. Int. J. Biol. Macromol. 2015, 81, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonhomme, O.; Leng, J.; Colin, A. Microfluidic wet-spinning of alginate microfibers: A theoretical analysis of fiber formation. Soft Matter 2012, 8, 10641–10649. [Google Scholar] [CrossRef]
- Jia, J.; Yao, D.; Wang, Y. Melt spinning of continuous fibers by cold air attenuation I: Experimental studies. Text. Res. J. 2014, 84, 593–603. [Google Scholar] [CrossRef]
- Gajjar, C.R.; King, M.W. Resorbable Fiber-Forming Polymers for Biotextile Applications; Springer International Publishing: New York, NY, USA, 2014. [Google Scholar]
- Imura, Y.; Hogan, R.M.C.; Jaffe, M. Dry spinning of synthetic polymer fibers. In Advances in Filament Yarn Spinning of Textiles and Polymers; Zhang, D., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 187–202. [Google Scholar]
- Lee, S.-H.; Park, S.-Y.; Choi, J.-H. Fiber Formation and Physical Properties of Chitosan Fiber Crosslinked by Epichlorohydrin in a Wet Spinning System: The effect of the Concentration of the Crosslinking Agent Epichlorohydrin. J. Appl. Polym. Sci. 2004, 92, 2054–2062. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; la Ossa, J.G.D.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Mirzaei, B.E.; Ramazani, S.A.A.; Shafiee, M.; Danaei, M. Studies on Glutaraldehyde Crosslinked Chitosan Hydrogel Properties for Drug Delivery Systems. Int. J. Polym. Mater. Polym. Biomater. 2013, 62, 605–611. [Google Scholar] [CrossRef]
- Blakeney, B.A.; Tambralli, A.; Anderson, J.M.; Andukuri, A.; Lim, D.-J.; Dean, D.R.; Jun, H.-W. Cell Infiltration and Growth in a Low Density, Uncompressed Three-Dimensional Electrospun Nanofibrous Scaffold. Biomaterials 2012, 32, 1583–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Choi, Y.-J.; Yi, H.-G.; Wang, J.H.; Cho, D.-W.; Jeong, Y.H. A cell-laden hybrid fiber/hydrogel composite for ligament regeneration with improved cell delivery and infiltration. Biomed. Mater. 2017, 12, 055010. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.M.; Kim, S.-E.; Van de Voorde, K.; Pokorski, J.K.; Korley, L.T.J. In Situ Fabrication of Fiber Reinforced Three-Dimensional Hydrogel Tissue Engineering Scaffolds. ACS Biomater. Sci. Eng. 2017, 3, 1869–1879. [Google Scholar] [CrossRef]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef] [Green Version]
- Sheffield, C.; Meyers, K.; Johnson, E.; Rajachar, R.M. Application of Composite Hydrogels to Control Physical Properties in Tissue Engineering and Regenerative Medicine. Gels 2018, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Regev, O.; Reddy, C.S.; Nseir, N.; Zussman, E. Hydrogel Reinforced by Short Albumin Fibers: Mechanical Characterization and Assessment of Biocompatibility. Macromol. Mater. Eng. 2012, 298, 283–291. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Oyen, M.L. Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea. J. Mech. Behav. Biomed. Mater. 2013, 21, 185–194. [Google Scholar] [CrossRef]
- Mohabatpour, F.; Karkhaneh, A.; Sharifi, A.M. A hydrogel/fiber composite scaffold for chondrocyte encapsulation in cartilage tissue regeneration. RSC Adv. 2016, 6, 83135–83145. [Google Scholar] [CrossRef]
- Xu, W.; Ma, J.; Jabbari, E. Material properties and osteogenic differentiation of marrow stromal cells on fiber-reinforced laminated hydrogel nanocomposites. Acta Biomater. 2010, 6, 1992–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, R.E.; Qu, X.; Jimenez-Vergara, A.C.; Bashur, C.A.; Guelcher, S.A.; Goldstein, A.S.; Hahn, M.S. Hydrogel–Electrospun Mesh Composites for Coronary Artery Bypass Grafts. Tissue Eng. Part C 2011, 17, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cho, B.; Martin, R.; Seu, M.; Zhang, C.; Zhou, Z.; Choi, J.S.; Jiang, X.; Chen, L.; Walia, G.; et al. Nanofiber-hydrogel composite–mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 2019, 11, eaau6210. [Google Scholar] [CrossRef] [PubMed]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. The three-dimensional vascularization of growth factor-releasing hybrid scaffold of poly (ε-caprolactone )/collagen fibers and hyaluronic acid hydrogel. Biomaterials 2011, 32, 8108–8117. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Johnson, J.; Lannutti, J.J.; Winter, J.O. Hydrogel–electrospun fiber composite materials for hydrophilic protein release. J. Control. Release 2012, 158, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Klicova, M.; Klapstova, A.; Chvojka, J.; Koprivova, B.; Jencova, V.; Horakova, J. Novel double-layered planar scaffold combining electrospun PCL fibers and PVA hydrogels with high shape integrity and water stability. Mater. Lett. 2020, 263, 127281. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Butcher, A.L.; Oyen, M.L. Strong and tough nanofibrous hydrogel composites based on biomimetic principles. Mater. Sci. Eng. C 2016, 72, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lee, J.; Seol, Y.-J.; Jeong, Y.H.; Cho, D.-W. Improving mechanical properties of alginate hydrogel by reinforcement with ethanol treated polycaprolactone nanofibers. Compos. Part B 2013, 45, 1216–1221. [Google Scholar] [CrossRef]
- Khorshidi, S.; Solouk, A.; Mirzadeh, H.; Mazinani, S.; Lagaron, J.M.; Shari, S.; Ramakrishna, S. A review of key challenges of electrospun scaffolds for tissue-engineering applications. J. Tissue Eng. Regen. Med. 2015, 10, 715–738. [Google Scholar] [CrossRef] [PubMed]
- Papaparaskeva, G.; Louca, M.; Voutouri, C.; Tanasă, E.; Stylianopoulos, T.; Krasia-Christoforou, T. Amalgamated Fiber/Hydrogel Composites Based on Semi-Interpenetrating Polymer Networks and Electrospun Nanocomposite Fibrous Mats. Eur. Polym. J. 2020, 140, 110041. [Google Scholar] [CrossRef]
- Hsieh, A.; Zahir, T.; Lapitsky, Y.; Amsden, B.; Wan, W.; Shoichet, M.S. Hydrogel/electrospun fiber composites influence neural stem/progenitor cell fate. Soft Matter 2010, 6, 2227–2237. [Google Scholar] [CrossRef] [Green Version]
- Ekaputra, A.K.; Prestwich, G.D.; Cool, S.M.; Hutmacher, D.W. Combining Electrospun Scaffolds with Electrosprayed Hydrogels Leads to Three-Dimensional Cellularization of Hybrid Constructs. Biomacromolecules 2008, 9, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Thorvaldsson, A.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L.; Gatenholm, P.; Walkenström, P. Development of Nanofiber-Reinforced Hydrogel Scaffolds for Nucleus Pulposus Regeneration by a Combination of Electrospinning and Spraying Technique. J. Appl. Polym. Sci. 2013, 128, 1158–1163. [Google Scholar] [CrossRef]
- Li, J.; Pan, K.; Tian, H.; Yin, L. The Potential of Electrospinning/Electrospraying Technology in the Rational Design of Hydrogel Structures. Macromol. Mater. Eng. 2020, 2000285, 1–26. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Wang, K.; Gu, X.; Luo, Y. Polymerization of Hydrogel Network on Microfiber Surface: Synthesis of Hybrid Water-Absorbing Matrices for Biomedical Applications. ACS Biomater. Sci. Eng. 2016, 6, 887–892. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Lu, Z.; Zhang, H.; Huang, J.; Yan, K.; Wang, D. Nanofiber-reinforced bulk hydrogel: Preparation and structural, mechanical and biological properties. J. Mater. Chem. B 2020, 8, 9794–9803. [Google Scholar] [CrossRef]
- Lin, S.; Cao, C.; Wang, Q.; Gonzalez, M.; Dolbow, J.E.; Zhao, X. Design of stiff, tough and stretchy hydrogel composites via nanoscale hybrid crosslinking and macroscale fiber reinforcement. Soft Matter 2014, 10, 7519–7527. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ye, Y.; Li, D.; Li, X.; Mu, C. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-PEG composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2015, 137, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Koh, W.-G. Composite Hydrogel of Methacrylated Hyaluronic Acid and Fragmented Polycaprolactone Nanofiber for Osteogenic Differentiation of Adipose-Derived Stem Cells. Pharmaceutics 2020, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, F.; Khorshidi, S.; Karkhaneh, A. A hydrogel/fiber scaffold based on silk fibroin/oxidized pectin with sustainable release of vancomycin hydrochloride. Eur. Polym. J. 2019, 118, 265–274. [Google Scholar] [CrossRef]
- Eslami, M.; Vrana, N.E.; Zorlutuna, P.; Sant, S.; Jung, S.; Masoumi, N.; Ramazan Ali, Khavari-Nejad; Javadi, G.; Khademhosseini, A. Fiber-reinforced hydrogel scaffolds for heart valve tissue engineering. J. Biomater. Appl. 2014, 29, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.R.; Diegelmann, R.F. What Makes Wounds Chronic. Surg. Clin. N. Am. 2020, 100, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, R.S.; Kandasubramanian, B. Advancements in nanofibers for wound dressing: A review. Eur. Polym. J. 2019, 117, 304–336. [Google Scholar] [CrossRef]
- Radhakumary, C.; Antonty, M.; Sreenivasan, K. Drug loaded thermoresponsive and cytocompatible chitosan based hydrogel as a potential wound dressing. Carbohydr. Polym. 2011, 83, 705–713. [Google Scholar] [CrossRef]
- Abbas, M.; Uçkay, I.; Lipsky, B.A. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin. Pharmacother. 2015, 16, 821–832. [Google Scholar] [CrossRef]
- Rubio-Elizalde, I.; Bernáldez-Sarabia, J.; Moreno-Ulloa, A.; Vilanova, C.; Juárez, P.; Licea-Navarro, A.; Castro-Cesenã, A.B. Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications. Carbohydr. Polym. 2018, 206, 455–467. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Homem, N.C.; Zille, A.; Amorim, M.T.P. Antimicrobial action and clotting time of thin, hydrated poly(vinyl alcohol )/cellulose acetate fi lms functionalized with LL37 for prospective wound-healing applications. J. Appl. Polym. Sci. 2019, 138, 48626. [Google Scholar]
- Zhao, X.; Sun, X.; Yildirimer, L.; Lang, Q.; Lin, Z.Y.; Zheng, R.; Zhang, Y.; Cui, W.; Annabi, N.; Khademhosseini, A. Cell infiltrative hydrogel fibrous scaffolds for accelerated wound healing. Acta Biomater. 2016, 49, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Lu, B.; Zhou, D.; Shao, M.; Xu, W.; Zhou, Y. Photocrosslinking maleilated hyaluronate/methacrylated poly(vinyl alcohol) nanofibrous mats for hydrogel wound dressings. Int. J. Biol. Macromol. 2019, 155, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechonol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Schulte, V.A.; Hahn, K.; Dhanasingh, A.; Heffels, K.-H.; Groll, J. Hydrogel–fibre composites with independent control over cell adhesion to gel and fibres as an integral approach towards a biomimetic artificial ECM. Biofabrication 2014, 6, 024106. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.A.; Nguyen, T.H.; Lee, B.-T. Preparation and characterization of electrospun PCL/PLGA membranes and chitosan/gelatin hydrogels for skin bioengineering applications. J. Mater. Sci. Mater. Med. 2011, 22, 2207–2218. [Google Scholar] [CrossRef]
- Sun, X.; Lang, Q.; Zhang, H.; Cheng, L.; Zhang, Y.; Pan, G. Electrospun Photocrosslinkable Hydrogel Fibrous Scaffolds for Rapid In Vivo Vascularized Skin Flap Regeneration. Adv. Funct. Mater. 2016, 27, 1604617. [Google Scholar] [CrossRef]
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun Polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2020, 409, 128144. [Google Scholar] [CrossRef]
- Azarniya, A.; Tamjid, E.; Eslahi, N.; Simchi, A. Modification of bacterial cellulose/keratin nanofibrous mats by a tragacanth gum-conjugated hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 134, 280–289. [Google Scholar] [CrossRef]
- Loo, Y.; Wong, Y.-C.; Cai, E.Z.; Ang, C.-H.; Raju, A.; Lakshmanan, A.; Koh, A.G.; Zhou, H.J.; Lim, T.-C.; Moochhala, S.M.; et al. Ultrashort peptide nanofibrous hydrogels for the acceleration of healing of burn wounds. Biomaterials 2014, 35, 4805–4814. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Gao, M.; Lin, J.; Wu, W.; Wang, J.; Chew, S.Y. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 2017, 7, 42212. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Ji, Y.; Chiu, A.; Lu, Y.-C.; Song, W.; Zhai, L.; Qi, L.; Luo, D.; Ma, M. Developing robust, hydrogel-based, nano fiber-enabled encapsulation devices (NEEDs ) for cell therapies. Biomaterials 2015, 37, 40–48. [Google Scholar] [CrossRef]
- Jain, K.K. An Overview of Drug Delivery Systems. In Methods in Molecular Biology; Jain, K.K., Ed.; Humana Press: New York, NY, USA, 2020; Volume 2059, pp. 1–54. [Google Scholar]
- Norouzi, M.; Shabani, I.; Ahvaz, H.H.; Soleimani, M. PLGA/gelatin hybrid nanofibrous scaffolds encapsulating EGF for skin regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 2225–2235. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Liu, L.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Torres-Martinez, E.J.; Bravo, J.M.C.; Medina, A.S.; González, G.L.P.; Gómez, L.J.V. A Summary of Electrospun Nanofibers as Drug Delivery System: Drugs Loaded and Biopolymers Used as Matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bas, O.; De-Juan-Pardo, E.M.; Catelas, I.; Hutmacher, D.W. The quest for mechanically and biologically functional soft biomaterials via soft network composites. Adv. Drug Deliv. Rev. 2018, 132, 214–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Rowley, J.A.; Eiselt, P.; Moy, E.M.; Bouhadir, K.H.; Mooney, D.J. Controlling mechanical and Swelling Properties of Alginate Hydrogels Independently by Cross-Linker Type and Cross-Linking Density. Macromolecules 2000, 33, 4291–4294. [Google Scholar] [CrossRef]
- Su, T.; Zhao, W.; Wu, L.; Dong, W.; Qi, X. Facile fabrication of functional hydrogels consisting of pullulan and polydopamine fibers for drug delivery. Int. J. Biol. Macromol. 2020, 163, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ghalei, S.; Nourmohammadi, J.; Solouk, A.; Mirzadeh, H. Enhanced Cellular Response Elicited by Addition of Amniotic fluid to Alginate Hydrogel-Electrospun Silk Fibroin Fibers for Potential Wound Dressing Application. Colloids Surfaces B Biointerfaces 2018, 172, 82–89. [Google Scholar] [CrossRef]
- Jaiswal, M.; Gupta, A.; Agrawal, A.K.; Jassal, M.; Dinda, A.K.; Koul, V. Bi-Layer Composite Dressing of Gelatin Nanofibrous Mat and Poly Vinyl Alcohol Hydrogel for Drug Delivery and Wound Healing Application: In-Vitro and In-Vivo Studies. J. Biomed. Nanotechnol. 2013, 9, 1495–1508. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, Y.; Wang, Q.; Li, D.; Hussain, T.; Ke, H.; Wei, Q. Mussel-inspired sandwich-like nanofibers/hydrogel composite with super adhesive, sustained drug release and anti-infection capacity. Chem. Eng. J. 2020, 399, 125668. [Google Scholar] [CrossRef]
| Polymer | Structural Formula | Origin/Synthesis Pathway | Main Characteristics | Known/Key/Main/Selected Applications | Reference | |
|---|---|---|---|---|---|---|
| Natural | Hyaluronic acid |  | Connective tissues of any vertebrate | Non-sulfated anionic glycosaminoglycan; linear conformation; hydrophilic; water-soluble; highly viscoelastic; non-immunogenic; biodegradable | Wound healing; biomolecule (e.g., ocatdecyl acrylate) delivery; cartilage/bone regeneration; bioink in 3D printing | [11,21,22,23,24] |
| Chitosan |  | Chitin (mostly found in the exoskeleton of shrimps, crabs, lobster and squid pens; cuticles of insects; and in lesser amounts, in cell walls of fungi, yeast and plants) | Cationic linear polysaccharide; hydrophilic; pH-dependent charge density; physicochemical properties dependent on the degree of acetylation, crystallinity, molecular weight and degradation; non-toxic; biodegradable; non-antigenic; biologically adhesive; hemostatic effect; antimicrobial; anti-inflammatory | Wound healing; bone/cartilage regeneration; antibiotic/antibacterial agents/growth factors delivery | [20,22,25,26,27] | |
| Alginate |  | Brown seaweed or bacteria (Azotobacter and Pseudomonas specie) | Anionic linear polysaccharide; slow gelation time; hydrophilic; water soluble; low toxicity; low cost; water retaining capacity; biodegradable | Wound dressings; burn treatments; protein/small chemical drug delivery; bone/cartilage regeneration | [5,28,29,30] | |
| Cellulose |  | Plants (mainly derived from cotton fiber, dried hemp and wood), bacteria (e.g., Acetobacter, Azotobacter, Rhizobium, Agrobacterium, Pseudomonas, Salmonella, Alcaligenes and Sarcina ventriculi species) | Linear homopolysaccharide; hydrophilic; rigid; fibrous morphology; relatively easy extraction; non-toxicity; low cost; biodegradable | Bone/tendon tissue regeneration; wound healing; loading antimicrobial agents and antibiotics | [31,32,33,34] | |
| Gelatin |  | Skin and bone of bovine and porcine, fish and marine organisms (incomplete denaturalization of collagen) | Linear polypeptide; hydrophilic; water soluble (35 °C); soluble in polyhydric alcohols and several other organic solvents; cost efficient; easily available; biodegradable; non-antigenic; similarity to collagen | Wound healing; bone regeneration; articular cartilage repair; tendon tissue engineering | [26,35,36,37] | |
| Collagen |  Collagen type I | Animals (e.g., Achilles tendon, bovine skin, porcine skin, and human cadaveric skin) | Polypeptide; good surface-active agent; enhanced water holding capacity; highly hydrophilic; twenty-eight different collagen types; low antigenic and cytotoxic responses; antioxidant; biodegradable; most abundant protein of animal origin | Wound healing; tissue replacement and regeneration (bone, cartilage, skin, blood vessels, trachea, esophagus); carriers for drug/protein delivery | [38,39,40,41] | |
| Synthetic | Poly(ethylene oxide) |  | Anionic ring-opening polymerization of ethylene oxide (EO) | Neutral polymer; hydrophilic; water soluble; low toxic; biodegradable | Gene/drug delivery systems; biomedical implants; neocartilage tissue formation; transdermal delivery | [42,43,44,45] |
| Poly(ε-caprolactone) |  | Ring-opening polymerization of ε-caprolactone monomer using a wide range of catalysts | Semicrystalline; hydrophobic; excellent mechanical strength; slow degradation rate; nontoxic; biodegradable | Tendon tissue engineering; skin regeneration; vascular scaffolds | [37,44,46,47] | |
| Polylactic acid |  | Polycondensation of lactic acid and ring opening polymerization of cyclic lactide | Thermoplastic aliphatic polyester; hydrophobic; poor ductility; low strength; bioabsorbable; biodegradable | Ligament and tendon repair; vascular stents; bone regeneration | [5,48,49,50] | |
| Poly(lactic-co-glycolic acid) |  | Ring-opening polymerization of lactide | Linear aliphatic copolymer; relatively hydrophobic; enhanced flexibility; thermal processibility; tunable degradation/biodegradation; minimal side effects | Wound healing; bone/ cardiac/periodontal tissue regeneration; protein/growth facto/antibiotic/ gene delivery | [51,52] | |
| Poly(vinylpyrrolidone) |  | Free radical polymerization from the vinylpyrrolidone monomer | Neutral polymer; amorphous; hydrophilic; water soluble; stable; nontoxic; adhesive power; non-biodegradable | Wound healing; gene delivery; biomedical implants (orthopedic, dental, vaginal, breast); neural/cardiac/pancreatic tissue regeneration | [17,53,54,55] | |
| Poly(vinyl alcohol) |  | Vinyl acetate with base catalyzed transesterification with ethanol | Linear polymer; hydrophilic; semicrystalline; water soluble; pH sensitive; high swelling capability; excellent chemo-thermal stability; transparency; high tensile; strength; high elongation at break; flexibility; non-toxic; non-carcinogenic and bioadhesive properties; non-biodegradable | Drugs/protein/growth factor/nanoparticle/gene delivery; skin healing and reconstruction; kidney regeneration | [48,56,57] | |
| Hydrogels Classification | |
|---|---|
| Source | Natural, synthetic or hybrid |
| Charge of polymers | Ionic, non-ionic, amphoteric or zwitterionic |
| Polymeric composition | Homopolymer, copolymer, multipolymer, IPN or semi-IPN |
| Configuration | Amorphous, crystalline or semicrystalline |
| Degradability | Biodegradable or non-biodegradable |
| Physical properties | Conventional or smart |
| Response | Physical, chemical, or biochemical/biological |
| Type of crosslinking | Chemical or physical |
| Hydrogels | Crosslinking Engine | Concept | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Physical | Ionic/Electrostatic Interaction | Interaction between a polyanion and a multivalent cation or a polycation, and vice versa (interaction between opposite charges) | Simple method; self-healing ability | Low stability in physiological environments and limited mechanical strength | [29,94,97,98] |
| Hydrogen Bonding | Hydrogen bond between polymer chains (electron-deficient hydrogen atom and a high electronegativity functional group) | Absence of chemical crosslinkers | High dilution and dispersion rate over a few hours in vivo | [85,99] | |
| Hydrophobic Interaction | Polymers with hydrophobic domains are capable of crosslinking in aqueous environments by means of reverse thermal gelation (“sol-gel”) (increased temperature leads to the aggregation of these domains) | Shape memory; autonomously self-healing properties; high degree of toughness | Poor mechanical properties | [99,100,101] | |
| Crystallization | The principle of freezing polymers at low temperatures, followed by thawing at room temperature causes the formation of crystals which leads to the formation of hydrogels | Stability and mechanical properties can be increased with increasing the freezing time and freeze–thaw cycles; simple method; not require additional chemicals and high temperature | Freeze/thaw processes applied for long periods of time can alter the behavior of the hydrogel | [96,102,103,104] | |
| Chemical | Photo-crosslinked | The crosslinking of monomers or oligomers is initiated in the presence of an irradiation of UV/visible light and a photoinitiator that, when absorbing photons, is cleaved and forms free radicals that trigger polymerization | No toxic crosslinking agents are required; excellent spatial and temporal selectivity; low processing cost and energy requirements | The photoinitiator can produce free radicals with effects on immunogenicity and cytotoxicity responses | [105,106] |
| Enzymatic Reaction | Certain enzymes (e.g., transglutaminases, horseradish peroxidase and tyrosinase) help to catalyze crosslinked reactions between two or more polymers | Mildness of the enzymatic reactions at normal physiological conditions; high efficiency; selectivity; non-toxicity; good biocompatibility; fast gelation process; tunable mechanical properties | Instability and poor availability of some of the enzymes | [107,108,109] | |
| Crosslinking Molecules | Crosslinkers (e.g., glutaraldehyde, carbodiimide agents, genipin and citric acid) are small molecules with two or more reactive functional groups responsible for the formation of bridges between polymers chains | Easiness and versatility method | Possible cytotoxicity of the crosslinking agent (e.g., glutaraldehyde) | [110,111,112] | |
| Polymer-Polymer | Crosslinking reaction occurs between pre-functionalized polymer chains with reactive functional groups under favorable conditions. Polymer–polymer bonds can be formed by Schiff bases and by Michael addition reactions | Not using crosslinking molecules | Requires the modification of the polymer chains before their conjugation | [113,114] |
| Hydrogels | Crosslinking Engine | Hydrogel Composition | Applications | Reference |
|---|---|---|---|---|
| Physical | Ionic Interaction | 6-PG-Na+-crosslinked CS | Drug delivery; wound dressing | [115] |
| CaCl2-crosslinked alginate-pectin | Wound dressing | [30] | ||
| Poloxamer-heparin/gellan gum | Bone marrow stem cells delivery | [117] | ||
| Al3+-crosslinked cellulose | Drug delivery | [118] | ||
| Hydrogen Bonding | PVA/poly(acrylic acid) | Surgical sutures and load-bearing fields | [119] | |
| 1,6-hexamethylenediamine (HMDA)-crosslinked cytosine and guanosine modified HA | Injectable drug delivery; soft tissue engineering; regenerative medicine | [120] | ||
| Crystallization | PVA/poly(ethylene glycol) | Wound dressing | [121] | |
| CS/PVA | Anti-inflammatory drug loading and release | [102] | ||
| PVA/cellulose | 2-layered skin model | [122] | ||
| Chemical | Photo-crosslinked | PEGDA | Tissue engineered heart valves | [123] |
| GelMA | Tissue engineering; drug delivery; regenerative medicine | [124] | ||
| GelMA/PEGDA | Bone regeneration | [116] | ||
| GelMA/CS | Tissue engineering | [60] | ||
| Enzymatic Reaction | Horseradish peroxidase -crosslinked HA/silk fibroin | Tissue engineering | [21] | |
| Horseradish peroxidase -crosslinked Silk fibroin- tyramine-substituted silk fibroin or gelatin | Cell delivery | [125] | ||
| Transglutaminase-crosslinked gelatin–laminin | Neuromuscular tissue engineering | [126] | ||
| Crosslinking Molecules | Genipin-crosslinked CS | Drug delivery systems in oral administration applications | [111] | |
| Genipin-crosslinked CS/gelatin | Drug delivery | [127] | ||
| Glutaraldehyde-crosslinked CS | Tissue engineering | [128] | ||
| Polymer–Polymer | CS/Alginate | Neuronal tissue engineering | [129] | |
| Hybrid | Chemical Crosslinking followed by Crystallization | Ethylene glycol diglycidyl ether-crosslinked microcrystalline Cellulose/PVA | Drug delivery | [130] |
| Type of Fibers | ||
|---|---|---|
| Natural | Plant | Bast fibers (e.g., jute and flax); seed fibers (e.g., cotton and coir); leaf fibers (e.g., banana and abaca); grass fibers (e.g., sugarcane bagasse and bamboo); straw fibers (e.g., rice, corn and wheat); wood fibers (e.g., softwood and hardwood) |
| Animal-Based | Wool; silk; hair | |
| Synthetic | Inorganic | Metals and alloys (e.g., metals fiber); metal or semi-metal compounds (e.g., glass and ceramics fibers); carbon-based fibers (e.g., carbon and graphene fibers) |
| Organic | Synthetic polymers (e.g., polyamide nylon, polyethylene terephthalate, phenol-formaldehyde, PVA, polycarbonate, polyvinyl chloride and polyolefins (polypropylene and polyethylene)); natural polymer (e.g., chitosan and alginate) | |
| Electrospinning | Wet-Spinning | Melt-Spinning | Dry-Spinning | |
|---|---|---|---|---|
| Set Up |  |  |  |  |
| Concept | The polymer dissolved in an appropriate solvent is injected by a needle towards a collection plate. Due to the high applied electric field, potential difference generated between the syringe (acts as an electrode) and the plate (acts as an electrode count), the polymer is attracted by the collecting plate, and the polymer solution is converted into nanofibers | The polymer is dissolved in an appropriate solvent and later injected through a fiery into a coagulation bath containing a non-solvent liquid. In the coagulation bath, continuous polymerization of the filaments occurs. After the formation of the fibers, they are extracted from the coagulation bath by means of rollers-induced capture | The solid polymer is heated above its melting point within the extruder and is then expelled through a die, solidifying on cooling. In a pick-up, the fibers are then recovered and mechanically stretched | The polymer is dissolved in a suitable solvent (must be highly volatile). The initial solution is injected through the spinneret and through a heating column that causes the solvent to evaporate. Consequently, the polymer solidifies, and dry fibers are attained |
| Advantages | Fibers with a large surface area, high porosity, great flexibility, and excellent mechanical properties; simple and straightforward process; cost efficiency | Wet-spun structures have greater intrinsic porosity and larger interconnected pores; versatile technique in terms of material selection | Fabrication process is quick; Not require added solvents | Enables spinning of polymers vulnerable to thermal degradation |
| Disadvantages | Fiber thickness increases density and reduces pore size in 3D structures that can limited the interaction of cells with the fibers; toxicity of the solvents and the instability of the jets; slow process | Long exposure to chemicals during the processing and coagulation may impact negatively on the cells’ microenvironments | Limited to thermically-resistant polymers; unstable in the production of fine fibers | Requires high temperatures which can affect the properties/characteristics of the fibers/fiber surface |
| Ref. | [164,165,166,167,168,169,170] | [171,172,173,174] | [175] | [176,177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, M.O.; Antunes, J.C.; Felgueiras, H.P. Recent Advances in Fiber–Hydrogel Composites for Wound Healing and Drug Delivery Systems. Antibiotics 2021, 10, 248. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10030248
Teixeira MO, Antunes JC, Felgueiras HP. Recent Advances in Fiber–Hydrogel Composites for Wound Healing and Drug Delivery Systems. Antibiotics. 2021; 10(3):248. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10030248
Chicago/Turabian StyleTeixeira, Marta O., Joana C. Antunes, and Helena P. Felgueiras. 2021. "Recent Advances in Fiber–Hydrogel Composites for Wound Healing and Drug Delivery Systems" Antibiotics 10, no. 3: 248. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10030248







