Impact of a Social Marketing Intervention on General Practitioners’ Antibiotic Prescribing Practices for Acute Respiratory Tract Complaints in Malta
Abstract
:1. Introduction
2. Results
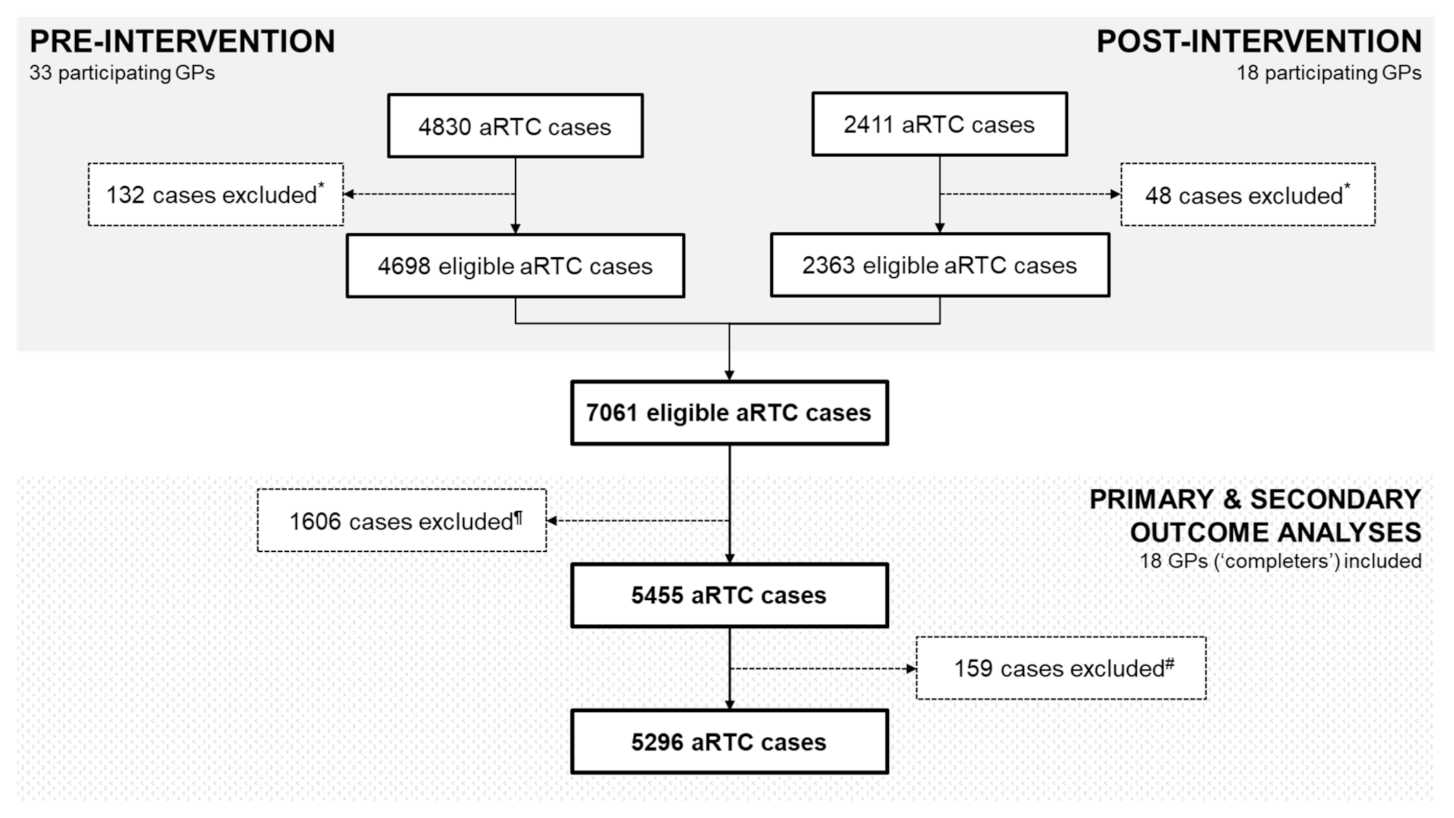
2.1. GP Participation and Reported aRTC Cases
2.2. GP Characteristics
2.3. Change in Overall Antibiotic Prescription Rates Pre- and Post-Intervention among 33 GPs (n = 7061 Eligible aRTC Cases)
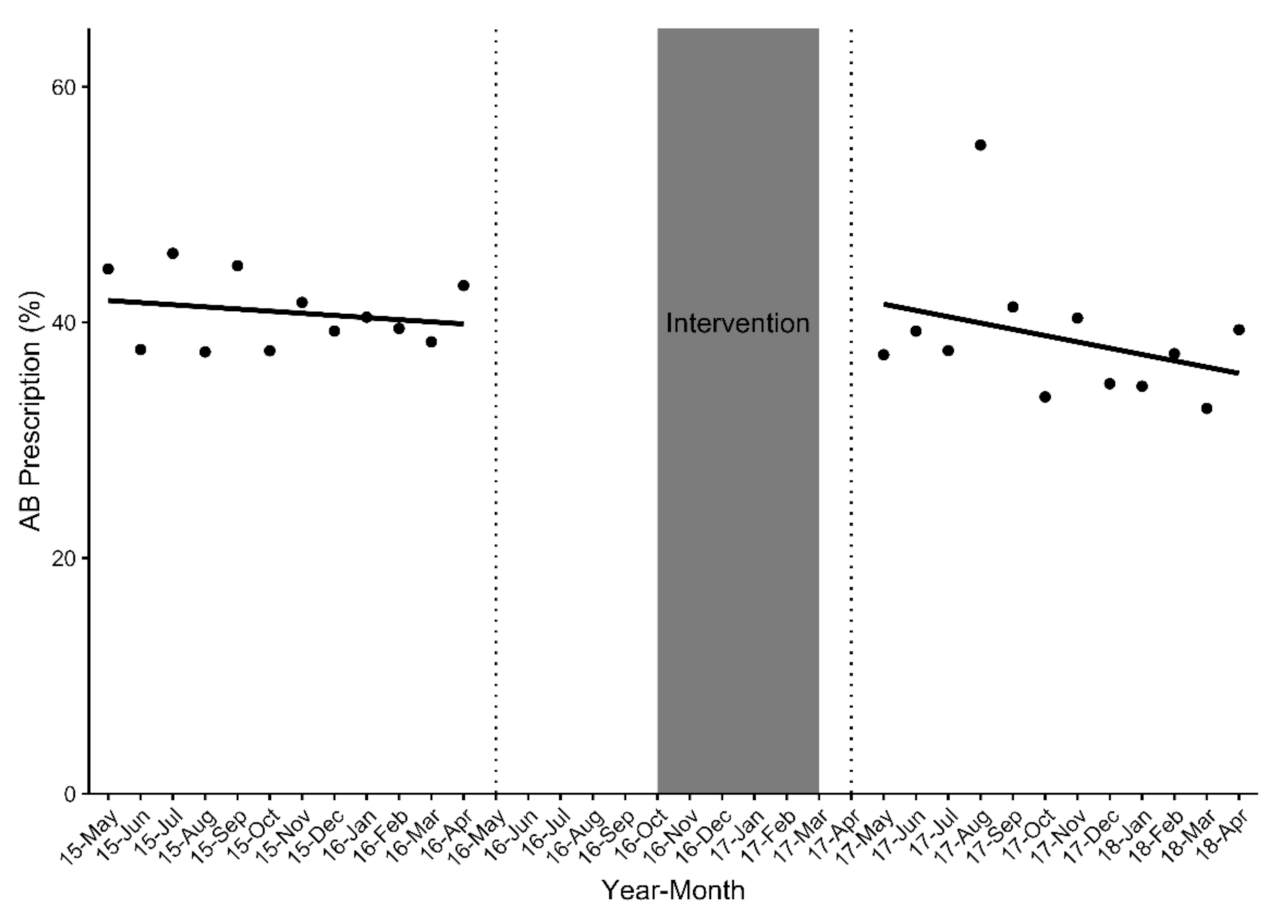
2.4. Impact of the SM Intervention on Antibiotic Prescription Rates among 18 GP ‘Completers’ (n = 5455 Eligible aRTC Cases)
2.4.1. Primary Outcome Analysis
2.4.2. Secondary Outcome Analysis: Antibiotic Prescription for Immediate Use among aRTC Cases Who Received an Antibiotic Prescription (n = 2150 Eligible aRTC Cases)
2.4.3. Secondary Outcome Analysis: DAP among aRTC Cases who Received an Antibiotic Prescription (n = 2150 Eligible aRTC Cases)
2.4.4. Secondary Outcome Analysis: Antibiotic Prescribing by Diagnosis among Eligible aRTC Cases Reported by GP ‘Completers’ (n = 5296 Eligible aRTC Cases)
2.4.5. Secondary Outcome Analysis: Antibiotic Prescribing in the Three Most Commonly Prescribed Antibiotic Classes (n = 2150 Eligible aRTC Cases)
3. Discussion
3.1. Summary of Findings
3.2. Impact of Multifaceted Interventions on Antibiotic Prescribing
3.3. Culture and Behaviour Change
3.4. Rapid Point-of-Care Tests
3.5. Delayed Antibiotic Prescription
3.6. Academic Detailing
3.7. Strengths and Limitations
4. Material and Methods
4.1. Study Design, Setting and Participants
4.1.1. Pre- and Post-Intervention Antibiotic Prescribing Surveillance
4.1.2. Intervention Development, Design, and Delivery
Patient Education Materials: Booklet and Posters
Antibiotic Prescribing Support Tools
GP Educational Sessions
Intervention Delivery
4.2. Data Analysis
4.2.1. Inclusion and Exclusion Criteria
4.2.2. Statistical Analyses
5. Conclusions
Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H.-P. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Goossens, H.; Ferech, M.; Stichele, R.V.; Elseviers, M. ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy for Containment of Antimicrobial Resistance; WHO: Geneva, Switzerland, 2001. [Google Scholar]
- European Centre for Disease Prevention and Control. Antimicrobial consumption in the EU/EEA, Annual Epidemiological Report for 2018; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Van Der Velden, A.W.; Kuyvenhoven, M.M.; Verheij, T.J.M. Improving antibiotic prescribing quality by an intervention embedded in the primary care practice accreditation: The ARTI4 randomized trial. J. Antimicrob. Chemother. 2015, 71, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroll, B. Antibiotics for upper respiratory tract infections: An overview of Cochrane reviews. Respir. Med. 2005, 99, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández Urrusuno, R.; Flores Dorado, M.; Vilches Arenas, A.; Serrano Martino, C.; Corral Baena, C.; Montero Balosa, M.C. Improving the appropriateness of antimicrobial use in primary care after implementation of a local antimicrobial guide in both levels of care. Eur. J. Clin. Pharm. 2014, 70, 1011–1020. [Google Scholar] [CrossRef]
- Shapiro, D.J.; Hicks, L.A.; Pavia, A.T.; Hersh, A.L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J. Antimicrob. Chemother. 2014, 69, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, S.L.; Gonzales, R.; Stahl, M.S.; Metlay, J.P. One size does not fit all: Evaluating an intervention to reduce antibiotic prescribing for acute bronchitis. BMC Health Serv. Res. 2013, 13, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- TNS Opinion & Social. Special Eurobarometer 478: Antimicrobial Resistance; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- TNS Opinion & Social. Special Eurobarometer 445: Antimicrobial Resistance; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- Saliba-Gustafsson, E.A.; Hampton, A.D.; Zarb, P.; Borg, M.A.; Stålsby Lundborg, C. Antibiotic prescribing for respiratory tract complaints in Malta: A 1 year repeated cross-sectional surveillance study. J. Antimicrob. Chemother. 2019, 74, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarb, P.; Borg, M.A. Consumption of antibiotics within ambulatory care in Malta. Malta Med. J. 2011, 23, 13–18. [Google Scholar]
- Stålsby Lundborg, C.; Tamhankar, A.J. Understanding and changing human behaviour—Antibiotic mainstreaming as an approach to facilitate modification of provider and consumer behaviour. Upsala J. Med. Sci. 2014, 119, 125–133. [Google Scholar] [CrossRef]
- Vinnard, C.; Linkin, D.R.; Localio, A.R.; Leonard, C.E.; Teal, V.L.; Fishman, N.O.; Hennessy, S. Effectiveness of interventions in reducing antibiotic use for upper respiratory infections in ambulatory care practices. Popul. Health Manag. 2013, 16, 22–27. [Google Scholar] [CrossRef]
- Arnold, S.R.; E Straus, S. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst. Rev. 2005, 2005, CD003539. [Google Scholar] [CrossRef]
- Ranji, S.R.; Steinman, M.A.; Shojania, K.G.; Gonzales, R. Interventions to reduce unnecessary antibiotic prescribing: A systematic review and quantitative analysis. Med. Care 2008, 46, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Edgar, T.; Boyd, S.D.; Palame, M.J. Sustainability for behaviour change in the fight against antibiotic resistance: A social marketing framework. J. Antimicrob. Chemother. 2009, 63, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, L.; Cairns, G.; Agnus, K.; Stead, M. Evidence Review: Social Marketing for the Prevention and Control of Communicable Disease; ECDC: Stockholm, Sweden, 2012. [Google Scholar]
- Edwards, R.; Charani, E.; Sevdalis, N.; Alexandrou, B.; Sibley, E.; Mullett, D.; Loveday, H.P.; Drumright, L.N.; Holmes, A. Optimisation of infection prevention and control in acute health care by use of behaviour change: A systematic review. Lancet Infect. Dis. 2012, 12, 318–329. [Google Scholar] [CrossRef]
- Duane, S.; Callan, A.; Galvin, S.; Murphy, A.; Domegan, C.; O’Shea, E.; Cormican, M.; Bennett, K.; O’Donnell, M.; Vellinga, A. Supporting the improvement and management of prescribing for urinary tract infections (SIMPle): Protocol for a cluster randomized trial. Trials 2013, 14, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formoso, G.; Paltrinieri, B.; Marata, A.M.; Gagliotti, C.; Pan, A.; Moro, M.L.; Capelli, O.; Magrini, N.; the LOCAAL Study Group. Feasibility and effectiveness of a low cost campaign on antibiotic prescribing in Italy: Community level, controlled, non-randomised trial. BMJ 2013, 347, f5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charani, E.; Edwards, R.; Sevdalis, N.; Alexandrou, B.; Sibley, E.; Mullett, D.; Franklin, B.D.; Holmes, A. Behavior change strategies to influence antimicrobial prescribing in acute care: A systematic review. Clin. Infect. Dis. 2011, 53, 651–662. [Google Scholar] [CrossRef] [Green Version]
- Dann, S. Redefining social marketing with contemporary commercial marketing definitions. J. Bus. Res. 2010, 63, 147–153. [Google Scholar] [CrossRef]
- Meeker, D.; Linder, J.A.; Fox, C.R.; Friedberg, M.W.; Persell, S.D.; Goldstein, N.J.; Knight, T.K.; Hay, J.W.; Doctor, J.N. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: A randomized clinical trial. JAMA 2016, 315, 562–570. [Google Scholar] [CrossRef]
- Bjerrum, L.; Munck, A.; Gahrn-Hansen, B.; Hansen, M.P.; Jarbol, D.E.; Cordoba, G.; Llor, C.; Cots, J.M.; Hernández, S.; López-Valcárcel, B.G.; et al. Health Alliance for prudent antibiotic prescribing in patients with respiratory tract infections (HAPPY AUDIT)-impact of a non-randomised multifaceted intervention programme. BMC Fam. Pract. 2011, 12, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonnell Norms Group. Antibiotic overuse: The influence of social norms. J. Am. Coll. Surg. 2008, 207, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Hofstede, G.; Hofstede, G.; Minkov, M. Cultures and Organisations: Software of the Mind; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Deschepper, R.; Grigoryan, L.; Lundborg, C.S.; Hofstede, G.; Cohen, J.; Van Der Kelen, G.; Deliens, L.; Haaijer-Ruskamp, F.M. Are cultural dimensions relevant for explaining cross-national differences in antibiotic use in Europe? BMC Health Serv. Res. 2008, 8, 123. [Google Scholar] [CrossRef]
- Borg, M.A. National cultural dimensions as drivers of inappropriate ambulatory care consumption of antibiotics in Europe and their relevance to awareness campaigns. J. Antimicrob. Chemother. 2011, 67, 763–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaygısız, Ü.; Lajunen, T.; Gaygısız, E. Socio-economic factors, cultural values, national personality and antibiotics use: A cross-cultural study among European countries. J. Infect. Public Health 2017, 10, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Saliba-Gustafsson, E.A.; Röing, M.; Borg, M.A.; Rosales-Klintz, S.; Stålsby Lundborg, C. General practitioners’ perceptions of delayed antibiotic prescription for respiratory tract infections: A phenomenographic study. PLoS ONE 2019, 14, e0225506. [Google Scholar] [CrossRef] [Green Version]
- Saliba-Gustafsson, E.A.; Nyberg, A.; Borg, M.A.; Rosales-Klintz, S.; Stålsby Lundborg, C. Barriers and facilitators to prudent antibiotic prescribing for acute respiratory tract infections: A qualitative study with general practitioners in Malta. PLoS ONE 2021, 16, e0246782. [Google Scholar] [CrossRef]
- Coenen, S.; Michiels, B.; Renard, D.; Denekens, J.; Van Royen, P. Antibiotic prescribing for acute cough: The effect of perceived patient demand. Br. J. Gen. Pract. 2006, 56, 183–190. [Google Scholar] [PubMed]
- Saliba-Gustafsson, E.A.; Hampton, A.D.; Zarb, P.; Orsini, N.; Borg, M.A.; Stålsby Lundborg, C. Factors associated with antibiotic prescribing in patients with acute respiratory tract complaints in Malta: A 1-year repeated cross-sectional surveillance study. BMJ Open 2019, 9, e032704. [Google Scholar] [CrossRef] [Green Version]
- Dekker, A.R.J.; Verheij, T.J.M.; Van Der Velden, A.W. Inappropriate antibiotic prescription for respiratory tract indications: Most prominent in adult patients. Fam. Pract. 2015, 32, 401–407. [Google Scholar] [CrossRef]
- Borg, M.A. Cultural determinants of infection control behaviour: Understanding drivers and implementing effective change. J. Hosp. Infect. 2014, 86, 161–168. [Google Scholar] [CrossRef]
- Malhotra-Kumar, S.; Lammens, C.; Coenen, S.; Van Herck, K.; Goossens, H. Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: A randomised, double-blind, placebo-controlled study. Lancet 2007, 369, 482–490. [Google Scholar] [CrossRef]
- Linder, J.A.; Stafford, R.S. Antibiotic treatment of adults with sore throat by community primary care physicians: A national survey, 1989–1999. JAMA 2001, 286, 1181–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, R.J.; Hoffman, J.R.; Bartlett, J.G.; Besser, R.E.; Gonzales, R.; Hickner, J.M.; Sande, M.A.; Centers for Disease Control and Prevention. Principles of appropriate antibiotic use for acute pharyngitis in adults: Background. Ann. Emerg. Med. 2001, 37, 711–719. [Google Scholar] [CrossRef]
- Spinks, A.; Glasziou, P.P.; Del Mar, C.B.; Spinks, A.B. Antibiotics for sore throat. Cochrane Database Syst. Rev. 2006, CD000023. [Google Scholar] [CrossRef] [Green Version]
- Little, P.; Williamson, I. Sore throat management in general practice. Fam. Pract. 1996, 13, 317–321. [Google Scholar] [CrossRef] [Green Version]
- Petersen, I.; Johnson, A.M.; Islam, A.; Duckworth, G.; Livermore, D.M.; Hayward, A.C. Protective effect of antibiotics against serious complications of common respiratory tract infections: Retrospective cohort study with the UK General Practice Research Database. BMJ 2007, 335, 982. [Google Scholar] [CrossRef] [Green Version]
- Gulliford, M.C.; Moore, M.V.; Little, P.; Hay, A.D.; Fox, R.; Prevost, A.T.; Juszczyk, D.; Charlton, J.; Ashworth, M. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: Cohort study using electronic health records. BMJ 2016, 354, i3410. [Google Scholar] [CrossRef] [Green Version]
- Anthierens, S.; Tonkin-Crine, S.; Douglas, E.; Fernandez-Vandellos, P.; Krawczyk, J.; Llor, C.; Frances, N.A.; Yardley, L.; Coenen, S.; Little, P.; et al. General practitioners’ views on the acceptability and applicability of a web-based intervention to reduce antibiotic prescribing for acute cough in multiple European countries: A qualitative study prior to a randomised trial. BMC Fam. Pract. 2012, 13, 101. [Google Scholar] [CrossRef] [Green Version]
- Llor, C.; Bjerrum, L.; Munck, A.; Cots, J.M.; Hernández, S.; Moragas, A.; Alcantara, J.D.D.; Alvarez, C.; Atienza, F.; Baeza, M.; et al. Access to point-of-care tests reduces the prescription of antibiotics among antibiotic-requesting subjects with respiratory tract infections. Respir. Care 2014, 59, 1918–1923. [Google Scholar] [CrossRef] [Green Version]
- Oppong, R.; Jit, M.; Smith, R.D.; Butler, C.C.; Melbye, H.; Mölstad, S.; Coast, J. Cost-effectiveness of point-of-care C-reactive protein testing to inform antibiotic prescribing decisions. Br. J. Gen. Pract. 2013, 63, e465–e471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llor, C.; Madurell, J.; Balagué-Corbella, M.; Gómez, M.; Cots, J.M. Impact on antibiotic prescription of rapid antigen detection testing in acute pharyngitis in adults: A randomised clinical trial. Br. J. Gen. Pract. 2011, 61, e244–e251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.; Rowbotham, S.; Chisholm, A.; Wearden, A.; Moschogianis, S.; Cordingley, L.; Baker, D.; Hyde, C.; Chew-Graham, C. Managing self-limiting respiratory tract infections: A qualitative study of the usefulness of the delayed prescribing strategy. Br. J. Gen. Pract. 2011, 61, e579–e589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Little, P.; Britten, N. Why do general practitioners prescribe antibiotics for sore throat? Grounded theory interview study. BMJ 2003, 326, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Høye, S.; Frich, J.C.; Lindbæk, M. Use and feasibility of delayed prescribing for respiratory tract infections: A questionnaire survey. BMC Fam. Pract. 2011, 12, 34. [Google Scholar] [CrossRef] [Green Version]
- Ryves, R.; Eyles, C.; Moore, M.; McDermott, L.; Little, P.; Leydon, G.M. Understanding the delayed prescribing of antibiotics for respiratory tract infection in primary care: A qualitative analysis. BMJ Open 2016, 6, e011882. [Google Scholar] [CrossRef] [Green Version]
- de la Poza Abad, M.; Mas Dalmau, G.; Moreno Bakedano, M.; González González, A.I.; Canellas Criado, Y.; Hernández Anadón, S.; Rotaeche Del Campo, R.; Torán Monserrat, P.; Negrete Palma, A.; Muñoz Ortiz, L.; et al. Prescription strategies in acute uncomplicated respiratory infections. JAMA Intern. Med. 2016, 176, 21–29. [Google Scholar] [CrossRef]
- Little, P.; Moore, M.; Kelly, J.; Williamson, I.; Leydon, G.; McDermott, L.; Mullee, M.; Stuart, B. On behalf of the PIPS Investigators Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: Pragmatic, factorial, randomised controlled trial. BMJ 2014, 348, g1606. [Google Scholar] [CrossRef] [Green Version]
- Dowell, J.; Pitkethly, M.; Bain, J.; Martin, S. A randomised controlled trial of delayed antibiotic prescribing as a strategy for managing uncomplicated respiratory tract infection in primary care. Br. J. Gen. Pract. 2001, 51, 200–205. [Google Scholar]
- Little, P.; Rumsby, K.; Kelly, J.; Watson, L.; Moore, M.; Warner, G.; Fahey, T.; Williamson, I. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: A randomized controlled trial. JAMA 2005, 293, 3029–3035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, P.; Gould, C.; Williamson, I.; Moore, M.; Warner, G.; Dunleavey, J. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. BMJ 2001, 322, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerts, A.F.; Scherpbier-de Haan, N.D.; de Koning, F.H.; van der Sterren, T.M.; van Weel, C.; Vervoort, G.M.; de Smet, P.A.; de Grauw, W.J. A pharmacy medication alert system based on renal function in older patients. Br. J. Gen. Pract. 2012, 62, e525–e529. [Google Scholar] [CrossRef] [Green Version]
- Dyrkorn, R.; Gjelstad, S.; Espnes, K.A.; Lindbæk, M. Peer academic detailing on use of antibiotics in acute respiratory tract infections. A controlled study in an urban Norwegian out-of-hours service. Scand. J. Prim. Health Care 2016, 34, 180–185. [Google Scholar] [CrossRef]
- Brax, H.; Fadlallah, R.; Al-Khaled, L.; Kahale, L.A.; Nas, H.; El-Jardali, F.; Akl, E.A. Association between physicians’ interaction with pharmaceutical companies and their clinical practices: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0175493. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.A.; Rogers, S.; Jamtvedt, G.; Oxman, A.D.; Odgaard-Jensen, J.; Kristoffersen, D.T.; Forsetlund, L.; Bainbridge, D.; Freemantle, N.; Davis, D.; et al. Educational outreach visits: Effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2007, 2007, CD000409. [Google Scholar] [CrossRef]
- Saliba-Gustafsson, E.A.; Borg, M.A.; Rosales-Klintz, S.; Nyberg, A.; Stålsby Lundborg, C. Maltese antibiotic stewardship programme in the community (MASPIC): Protocol of a prospective quasiexperimental social marketing intervention. BMJ Open 2017, 7, e017992. [Google Scholar] [CrossRef]
- Government of Malta. National Antibiotic Committee. Available online: https://deputyprimeminister.gov.mt/en/nac/Pages/nac.aspx (accessed on 8 January 2021).
- World Health Organization. Collaborating Centre for Drug Statistics Methodology, Anatomical Therapeutic Chemical Classification with Defined Daily Doses. Available online: https://www.whocc.no/atc_ddd_index/ (accessed on 8 January 2021).


| GP Characteristics | Pre-Intervention (n = 33) | Post-Intervention (n = 18) | p-Value |
|---|---|---|---|
| Age, median (IQR) | 52 (42–57) | 46 (41–53) | 0.221 |
| Sex, n Male/Female | 24/9 | 13/5 | 0.969 |
| Years of GP practice, median (IQR) | 23 (16–29) | 20 (15–24) | 0.121 |
| Type of practice, n # Group/Solo | 15/18 | 10/8 | 0.490 |
| Employment sector, n Public only Private only Public & private | 11 20 2 | 8 9 1 | 0.791 |
| Employment type, n Full-time/Part-time | 22/11 | 14/4 | 0.527 |
| GP Characteristics | Completers (n = 18) | Non-Completers (n = 15) | p-Value |
|---|---|---|---|
| Age, median (IQR) | 46 (41–53) | 55 (48–59) | 0.025 |
| Sex, n Male/Female | 13/5 | 11/4 | 1.000 |
| Years of GP practice, median (IQR) | 20 (15–24) | 29 (26–33) | 0.004 |
| Type of practice, n # Group/Solo | 10/8 | 5/10 | 0.172 |
| Employment sector, n Public only Private only Public & private | 8 9 1 | 3 11 1 | 0.407 |
| Employment type, n Full-time/Part-time | 14/4 | 8/7 | 0.163 |
| Antibiotic prescription (pre-intervention) *, n (%) | 1260 (40.8%) | 892 (55.5%) | 0.000 |
| Outcome | Baseline Indicator Estimate | Baseline Trend * | Short-Term Effect * | Long-Term Effect * | Post-Intervention Linear Trend * |
|---|---|---|---|---|---|
| n (%) [95% CI] | _t | _x13 | _x_t13 | _b[_t]+_b[_x_t13] | |
| Antibiotic prescription | 1295 (41.87) [40.11–43.62] | −0.18 [−0.51–0.15] | 1.86 [−6.16–9.89] | −0.35 [−1.16–0.45] | −0.53 [−1.34–0.27] |
| Immediate antibiotic prescription | 985 (78.21) [74.22–82.19] | −0.22 [−0.70–0.26] | −6.84 [−15.77–2.09] | 0.14 [−0.86–1.15] | −0.08 [−1.04–0.88] |
| Delayed antibiotic prescription | 275 (21.79) [17.81–25.78] | 0.22 [−0.26–0.70] | 6.84 [−2.09–15.77] | −0.14 [−1.15–0.86] | 0.08 [−0.88–1.04] |
| Diagnosis (n) | Antibiotic Prescriptions (Pre) n/N (%) | Antibiotic Prescriptions (Post) n/N (%) | OR | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| Tonsillitis (n = 453) | 251/267 | (94.0) | 176/186 | (94.6) | 1.06 | 0.49–2.33 | 0.876 |
| Bronchitis (n = 585) | 267/314 | (85.0) | 216/271 | (79.7) | 0.71 | 0.48–1.05 | 0.084 |
| Otitis media (n = 125) | 69/75 | (92.0) | 39/50 | (78.0) | 0.38 | 0.15–0.97 | 0.044 |
| Pharyngitis (n = 812) | 208/417 | (49.9) | 220/395 | (55.7) | 1.41 | 1.07–1.86 | 0.015 |
| Sinusitis (n = 342) | 106/190 | (55.8) | 64/152 | (42.1) | 0.60 | 0.42–0.85 | 0.004 |
| Exacerbation ¶ (n = 288) | 87/180 | (48.3) | 38/106 | (35.8) | 0.65 | 0.42–1.00 | 0.051 |
| Influenza (n = 231) | 35/105 | (33.3) | 23/126 | (18.3) | 0.59 | 0.33–1.06 | 0.076 |
| Allergy (n = 264) | 12/163 | (7.4) | 10/101 | (10.0) | 1.53 | 0.48–4.93 | 0.472 |
| Common cold (n = 1952) | 110/1145 | (9.6) | 37/807 | (4.6) | 0.51 | 0.36–0.73 | 0.000 |
| Diagnoses (n) | J01C (Pre) | J01C (Post) | OR | 95% CI | p-Value | J01D (Pre) | J01D (Post) | OR | 95% CI | p-Value | J01F (Pre) | J01F (Post) | OR | 95% CI | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||||||||||||||
| Tonsillitis (n = 453) | 151 | (56.6) | 120 | (64.5) | 1.43 | 0.97–2.09 | 0.067 | 45 | (16.9) | 25 | (13.4) | 0.70 | 0.41–1.17 | 0.173 | 55 | (20.6) | 31 | (16.7) | 0.78 | 0.48–1.27 | 0.315 | |
| Bronchitis (n = 585) | 118 | (37.6) | 116 | (42.8) | 1.00 | 0.74–1.36 | 0.999 | 35 | (11.1) | 17 | (6.3) | 0.53 | 0.29–0.97 | 0.040 | 84 | (26.8) | 63 | (23.2) | 1.10 | 0.75–1.62 | 0.625 | |
| Otitis media (n = 125) | 42 | (56.0) | 21 | (42.0) | 0.63 | 0.31–1.29 | 0.207 | 13 | (17.3) | 8 | (16.0) | 0.94 | 0.42–2.09 | 0.873 | 14 | (18.7) | 10 | (20.0) | 0.91 | 0.47–1.75 | 0.775 | |
| Pharyngitis (n = 812) | 88 | (21.1) | 98 | (24.8) | 1.29 | 0.97–1.72 | 0.079 | 44 | (10.6) | 33 | (8.4) | 0.77 | 0.48–1.22 | 0.263 | 71 | (17.0) | 86 | (21.8) | 1.46 | 1.01–2.12 | 0.046 | |
| Sinusitis (n = 342) | 37 | (19.5) | 33 | (21.7) | 1.42 | 0.88–2.30 | 0.154 | 31 | (16.3) | 6 | (3.9) | 0.16 | 0.06–0.46 | 0.001 | 34 | (17.9) | 19 | (12.5) | 0.70 | 0.39–1.24 | 0.221 | |
| Exacerbation ¶ (n = 288) | 45 | (25.0) | 24 | (22.6) | 0.79 | 0.47–1.34 | 0.386 | 16 | (8.9) | 3 | (2.8) | 0.37 | 0.11–1.21 | 0.099 | 16 | (8.9) | 4 | (3.8) | 0.46 | 0.15–1.36 | 0.159 | |
| Influenza (n = 231) | 8 | (7.6) | 10 | (7.9) | 1.07 | 0.40–2.88 | 0.895 | 7 | (6.7) | 5 | (4.0) | 0.65 | 0.15–2.93 | 0.582 | 19 | (18.1) | 6 | (4.8) | 0.27 | 0.08–0.89 | 0.032 | |
| Allergy (n = 264) | 5 | (3.1) | 8 | (7.9) | 4.01 | 0.48–33.49 | 0.200 | 2 | (1.2) | 0 | (0.0) | - | - | - | 5 | (3.1) | 1 | (1.0) | 0.29 | 0.03–3.04 | 0.303 | |
| Common cold (n = 1952) | 52 | (4.5) | 20 | (2.5) | 0.59 | 0.37–0.92 | 0.021 | 26 | (2.3) | 8 | (1.0) | 0.45 | 0.20–1.01 | 0.054 | 31 | (2.7) | 8 | (1.0) | 0.39 | 0.18–0.84 | 0.016 | |
| Antibiotic Class | N (%) | Pre-Intervention | Post-Intervention | OR | 95% CI | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | ||||||||
| J01A tetracyclines | 29 | (1.3) | 9 | (0.7) | 20 | (2.2) | 3.45 | 1.55-7.69 | 0.002 |
| J01C β-lactam antibacterials, penicillins | 1064 | (49.5) | 590 | (46.8) | 474 | (53.3) | 1.20 | 1.02-1.42 | 0.032 |
| J01D other β-lactam antibacterials | 359 | (16.7) | 242 | (19.2) | 117 | (13.1) | 0.61 | 0.48-0.78 | 0.000 |
| J01F macrolides | 633 | (29.4) | 375 | (29.8) | 258 | (29.0) | 1.05 | 0.87-1.27 | 0.617 |
| J01M quinolones | 74 | (3.4) | 50 | (4.0) | 24 | (2.7) | 0.75 | 0.45-1.24 | 0.266 |
| TOTAL antibiotic prescriptions * | 2150 | (100.00) | 1260 | (100.0) | 890 | (100.0) | 0.88 | 0.80-0.98 | 0.016 |
| Intervention Components * | Process Indicators | ||||
|---|---|---|---|---|---|
| Educational sessions | Session 1 | Session 2 | Session 3 | Session 4 | |
| Duration of session, hrs | 2 | 2 | 2 | 2 | |
| Attendance in person, % | 50 | 54 | 38 | 42 | |
| Attendance online (live), % | 4 | 8 | 8 | 8 | |
| Attendance online (recorded), % | 13 | 8 | 17 | 8 | |
| Attendance (total), % | 67 | 71 | 63 | 58 | |
| Waiting room posters | No. of poster sets printed and disseminated | 41 | |||
| Patient booklets | No. of booklets printed and disseminated | 8600 | |||
| National antibiotic guidelines | No. of guidelines printed and disseminated | 24 | |||
| DAP pads # | No. of DAPs printed (total) and disseminated | 5700 | |||
| No. of DAP pads disseminated | 190 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machowska, A.; Marrone, G.; Saliba-Gustafsson, P.; Borg, M.A.; Saliba-Gustafsson, E.A.; Stålsby Lundborg, C. Impact of a Social Marketing Intervention on General Practitioners’ Antibiotic Prescribing Practices for Acute Respiratory Tract Complaints in Malta. Antibiotics 2021, 10, 371. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10040371
Machowska A, Marrone G, Saliba-Gustafsson P, Borg MA, Saliba-Gustafsson EA, Stålsby Lundborg C. Impact of a Social Marketing Intervention on General Practitioners’ Antibiotic Prescribing Practices for Acute Respiratory Tract Complaints in Malta. Antibiotics. 2021; 10(4):371. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10040371
Chicago/Turabian StyleMachowska, Anna, Gaetano Marrone, Peter Saliba-Gustafsson, Michael A. Borg, Erika A. Saliba-Gustafsson, and Cecilia Stålsby Lundborg. 2021. "Impact of a Social Marketing Intervention on General Practitioners’ Antibiotic Prescribing Practices for Acute Respiratory Tract Complaints in Malta" Antibiotics 10, no. 4: 371. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10040371






