Exploring the Contribution of the AcrB Homolog MdtF to Drug Resistance and Dye Efflux in a Multidrug Resistant E. coli Isolate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Impact of mdtF Inactivation on Drug Susceptibility
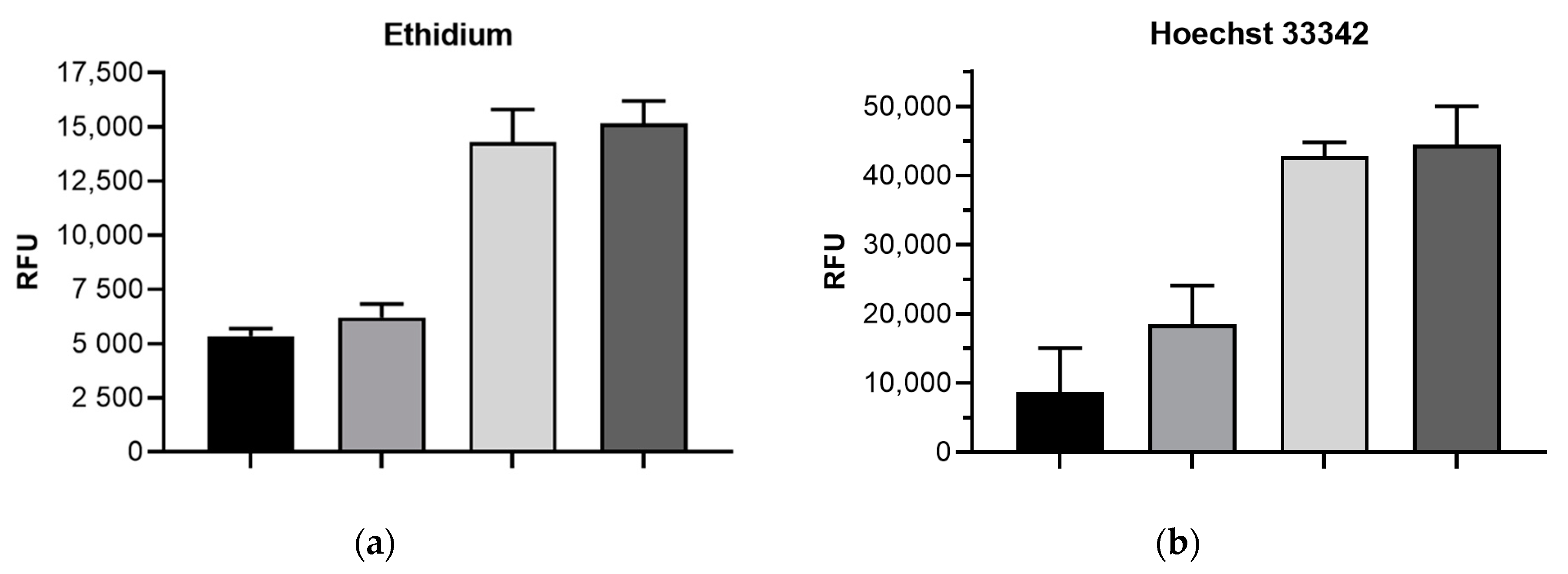
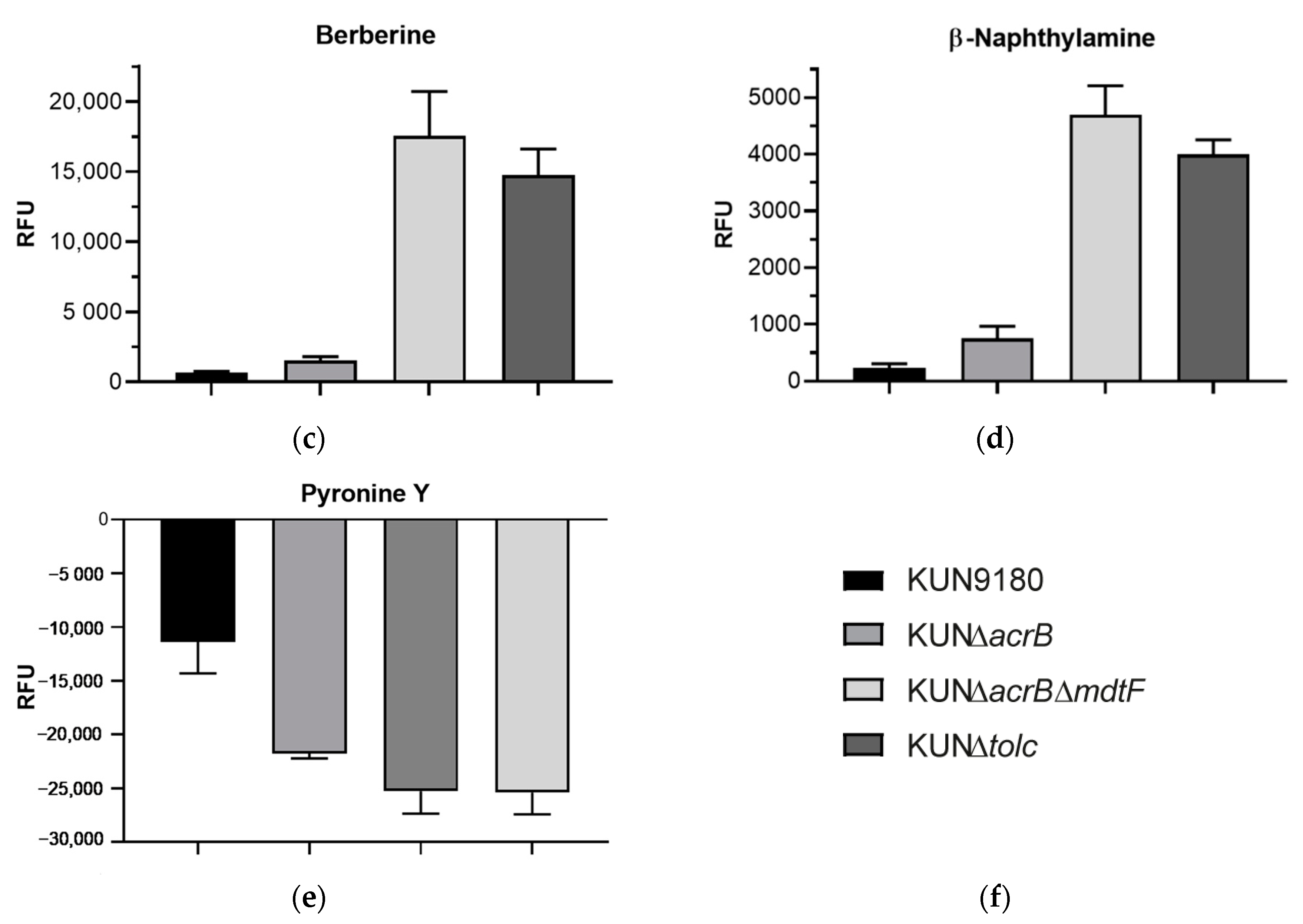
2.2. Impact of mdtF Inactivation on Intracellular Dye Accumulation
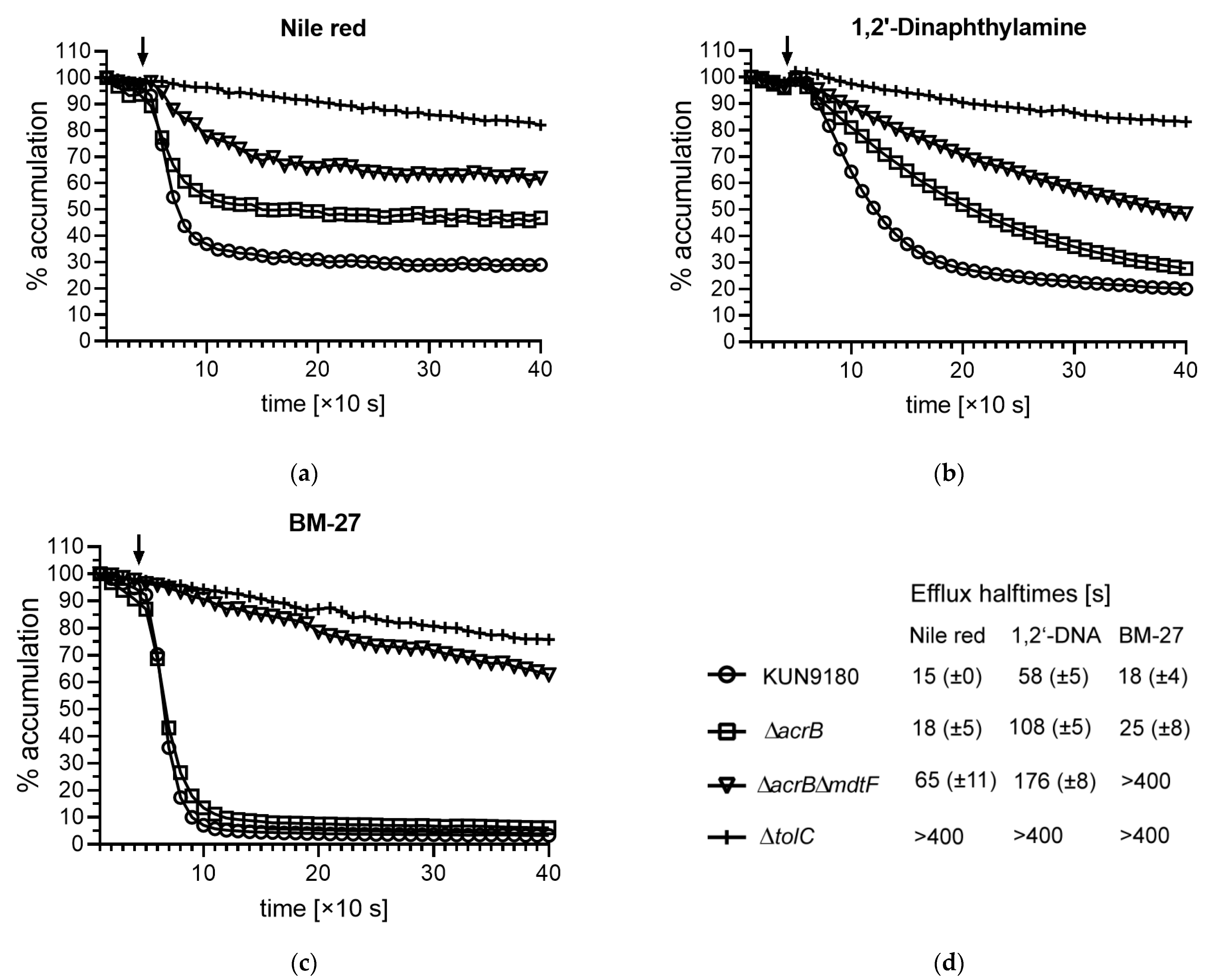
2.3. Impact of mdtF Inactivation on Real-Time Efflux
3. Materials and Methods
3.1. Strains, Growth Conditions, Chemicals
3.2. Susceptibility Testing
3.3. Engineering of Mutant KUN∆acrB∆mdtF
3.4. Dye Accumulation Assays
3.5. Real-Time Efflux Assays
3.6. Statistical Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Kern, W.V.; Oethinger, M.; Jellen-Ritter, A.S.; Levy, S.B. Non-Target Gene Mutations in the Development of Fluoroquinolone Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2000, 44, 814–820. [Google Scholar] [CrossRef] [Green Version]
- Schuster, S.; Vavra, M.; Schweigger, T.M.; Rossen, J.W.; Matsumura, Y.; Kern, W.V. Contribution of AcrAB-TolC to multidrug resistance in an Escherichia coli sequence type 131 isolate. Int. J. Antimicrob. Agents 2017, 50, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Cunrath, O.; Meinel, D.M.; Maturana, P.; Fanous, J.; Buyck, J.M.; Auguste, P.S.; Seth-Smith, H.M.; Körner, J.; Dehio, C.; Trebosc, V.; et al. Quantitative contribution of efflux to multi-drug resistance of clinical Escherichia coli and Pseudomonas aeruginosa strains. EBioMedicine 2019, 41, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Bialek-Davenet, S.; Lavigne, J.-P.; Guyot, K.; Mayer, N.; Tournebize, R.; Brisse, S.; Leflon-Guibout, V.; Nicolas-Chanoine, M.-H. Differential contribution of AcrAB and OqxAB efflux pumps to multidrug resistance and virulence in Klebsiella pneumoniae. J. Antimicrob. Chemother. 2015, 70, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravey, F.; Cattoir, V.; Ethuin, F.; Fabre, L.; Beyrouthy, R.; Bonnet, R.; Le Hello, S.; Guérin, F. ramR deletion in an Enterobacter hormaechei isolate as a consequence of therapeutic failure to key antibiotics in a long-term hospitalized patient. Antimicrob. Agents Chemother. 2020, 64. [Google Scholar] [CrossRef]
- Ruzin, A.; Immermann, F.W.; Bradford, P.A. RT-PCR and Statistical Analyses of adeABC Expression in Clinical Isolates of Acinetobacter calcoaceticus–Acinetobacter baumannii Complex. Microb. Drug Resist. 2010, 16, 87–89. [Google Scholar] [CrossRef]
- Gajdács, M. Carbapenem-Resistant but Cephalosporin-Susceptible Pseudomonas aeruginosa in Urinary Tract Infections: Opportunity for Colistin Sparing. Antibiotics 2020, 9, 153. [Google Scholar] [CrossRef] [Green Version]
- Bohnert, J.A.; Schuster, S.; Fähnrich, E.; Trittler, R.; Kern, W.V. Altered spectrum of multidrug resistance associated with a single point mutation in the Escherichia coli RND-type MDR efflux pump YhiV (MdtF). J. Antimicrob. Chemother. 2006, 59, 1216–1222. [Google Scholar] [CrossRef]
- Camp, J.; Schuster, S.; Vavra, M.; Schweigger, T.; Rossen, J.W.A.; Reuter, S.; Kern, W.V. Limited Multidrug Resistance Efflux Pump Overexpression among Multidrug-Resistant Escherichia coli Strains of ST131. Antimicrob. Agents Chemother. 2021, 65, 01735-20. [Google Scholar] [CrossRef]
- Schuster, S.; Vavra, M.; Köser, R.; Rossen, J.W.A.; Kern, W.V. New Topoisomerase Inhibitors: Evaluating the Potency of Gepotidacin and Zoliflodacin in Fluoroquinolone-Resistant Escherichia coli upon tolC Inactivation and Differentiating Their Efflux Pump Substrate Nature. Antimicrob. Agents Chemother. 2020, 65. [Google Scholar] [CrossRef] [PubMed]
- Nishino, K.; Yamaguchi, A. Analysis of a Complete Library of Putative Drug Transporter Genes in Escherichia coli. J. Bacteriol. 2001, 183, 5803–5812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zgurskaya, H.I.; Krishnamoorthy, G.; Ntreh, A.; Lu, S. Mechanism and Function of the Outer Membrane Channel TolC in Multidrug Resistance and Physiology of Enterobacteria. Front. Microbiol. 2011, 2, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleuel, C.; Grosse, C.; Taudte, N.; Scherer, J.; Wesenberg, D.; Krauß, G.J.; Nies, D.H.; Grass, G. TolC Is Involved in Enterobactin Efflux across the Outer Membrane of Escherichia coli. J. Bacteriol. 2005, 187, 6701–6707. [Google Scholar] [CrossRef] [Green Version]
- Vega, D.E.; Young, K.D. Accumulation of periplasmic enterobactin impairs the growth and morphology of Escherichia coli tolC mutants. Mol. Microbiol. 2013, 91, 508–521. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, M.; Horiyama, T.; Zhang, Y.; Li, X.; Nishino, K.; Yan, A. The Multidrug Efflux Pump MdtEF Protects against Nitrosative Damage during the Anaerobic Respiration in Escherichia coli. J. Biol. Chem. 2011, 286, 26576–26584. [Google Scholar] [CrossRef] [Green Version]
- Sjuts, H.; Vargiu, A.V.; Kwasny, S.M.; Nguyen, S.T.; Kim, H.-S.; Ding, X.; Ornik, A.R.; Ruggerone, P.; Bowlin, T.L.; Nikaido, H.; et al. Molecular basis for inhibition of AcrB multidrug efflux pump by novel and powerful pyranopyridine derivatives. Proc. Natl. Acad. Sci. USA 2016, 113, 3509–3514. [Google Scholar] [CrossRef] [Green Version]
- Lomovskaya, O.; Warren, M.S.; Lee, A.; Galazzo, J.; Fronko, R.; Lee, M.; Blais, J.; Cho, D.; Chamberland, S.; Renau, T.; et al. Identification and Characterization of Inhibitors of Multidrug Resistance Efflux Pumps in Pseudomonas aeruginosa: Novel Agents for Combination Therapy. Antimicrob. Agents Chemother. 2001, 45, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Wang-Kan, X.; Rodríguez-Blanco, G.; Southam, A.D.; Winder, C.L.; Dunn, W.B.; Ivens, A.; Piddock, L.J.V. Metabolomics Reveal Potential Natural Substrates of AcrB in Escherichia coli and Salmonella enterica Serovar Typhimurium. mBio 2021, 12. [Google Scholar] [CrossRef]
- Bohnert, J.A.; Karamian, B.; Nikaido, H. Optimized Nile Red Efflux Assay of AcrAB-TolC Multidrug Efflux System Shows Competition between Substrates. Antimicrob. Agents Chemother. 2010, 54, 3770–3775. [Google Scholar] [CrossRef] [Green Version]
- Bohnert, J.A.; Schuster, S.; Szymaniak-Vits, M.; Kern, W.V. Determination of Real-Time Efflux Phenotypes in Escherichia coli AcrB Binding Pocket Phenylalanine Mutants Using a 1,2′-Dinaphthylamine Efflux Assay. PLoS ONE 2011, 6, e21196. [Google Scholar] [CrossRef] [Green Version]
- Bohnert, J.A.; Schuster, S.; Kern, W.V.; Karcz, T.; Olejarz, A.; Kaczor, A.; Handzlik, J.; Kieć-Kononowicz, K. Novel Piperazine Arylideneimidazolones Inhibit the AcrAB-TolC Pump in Escherichia coli and Simultaneously Act as Fluorescent Membrane Probes in a Combined Real-Time Influx and Efflux Assay. Antimicrob. Agents Chemother. 2016, 60, 1974–1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; A Shoemaker, B.; A Thiessen, P.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Schumacher, A.; Steinke, P.; Bohnert, J.A.; Akova, M.; Jonas, D.; Kern, W.V. Effect of 1-(1-naphthylmethyl)-piperazine, a novel putative efflux pump inhibitor, on antimicrobial drug susceptibility in clinical isolates of Enterobacteriaceae other than Escherichia coli. J. Antimicrob. Chemother. 2005, 57, 344–348. [Google Scholar] [CrossRef] [Green Version]
- Schuster, S.; Kohler, S.; Buck, A.; Dambacher, C.; König, A.; Bohnert, J.A.; Kern, W.V. Random Mutagenesis of the Multidrug Transporter AcrB from Escherichia coli for Identification of Putative Target Residues of Efflux Pump Inhibitors. Antimicrob. Agents Chemother. 2014, 58, 6870–6878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, S.; Bohnert, J.A.; Vavra, M.; Rossen, J.W.; Kern, W.V. Proof of an Outer Membrane Target of the Efflux Inhibitor Phe-Arg-β-Naphthylamide from Random Mutagenesis. Molecules 2019, 24, 470. [Google Scholar] [CrossRef] [Green Version]
| MIC in µg/mL 1 | |||
|---|---|---|---|
| E. coli Strain/Mutant | Nadifloxacin | Zoliflodacin | Novobiocin |
| KUN9180 | >512 (0) | 4 (0) | 128 (64) |
| KUN∆acrB | 32 (16) | 0.25 (0) | 4 (2) |
| KUN∆acrB∆mdtF | 16 (0) | 0.125 (0) | 2 (0) |
| KUN∆tolC | 4 (2) | 0.045 (0.02) | 1 (0) |
| Oligonucleotide | Sequence (5′-3′) 1 | Application |
|---|---|---|
| upOl-mdtF-FRT-PGKgb2neo | gtcactcaggtgattgagcaaaatatgaatgggcttgatggcctgatgtaaattaaccctcactaaagggcg | FRT-PGK-gb2neo-FRTcassette amplification |
| lowOl-mdtF-FRT-PGKgb2neo | gcggtgccatcgtgccagaggcgttgcgtacataccattggttgatgttataatacgactcactatagggctc | FRT-PGK-gb2neo-FRTcassette amplification |
| Check-mdtF-fw | ggcgatcatgaacttaccgg | Check-primer for mdtF insertions |
| Check-mdtF-rv | ggatgccgttgtagcgttc | Check-primer for mdtF insertions |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schuster, S.; Vavra, M.; Greim, L.; Kern, W.V. Exploring the Contribution of the AcrB Homolog MdtF to Drug Resistance and Dye Efflux in a Multidrug Resistant E. coli Isolate. Antibiotics 2021, 10, 503. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050503
Schuster S, Vavra M, Greim L, Kern WV. Exploring the Contribution of the AcrB Homolog MdtF to Drug Resistance and Dye Efflux in a Multidrug Resistant E. coli Isolate. Antibiotics. 2021; 10(5):503. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050503
Chicago/Turabian StyleSchuster, Sabine, Martina Vavra, Ludwig Greim, and Winfried V. Kern. 2021. "Exploring the Contribution of the AcrB Homolog MdtF to Drug Resistance and Dye Efflux in a Multidrug Resistant E. coli Isolate" Antibiotics 10, no. 5: 503. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050503






