Phage PPPL-1, A New Biological Agent to Control Bacterial Canker Caused by Pseudomonas syringae pv. actinidiae in Kiwifruit
Abstract
:1. Introduction
2. Results
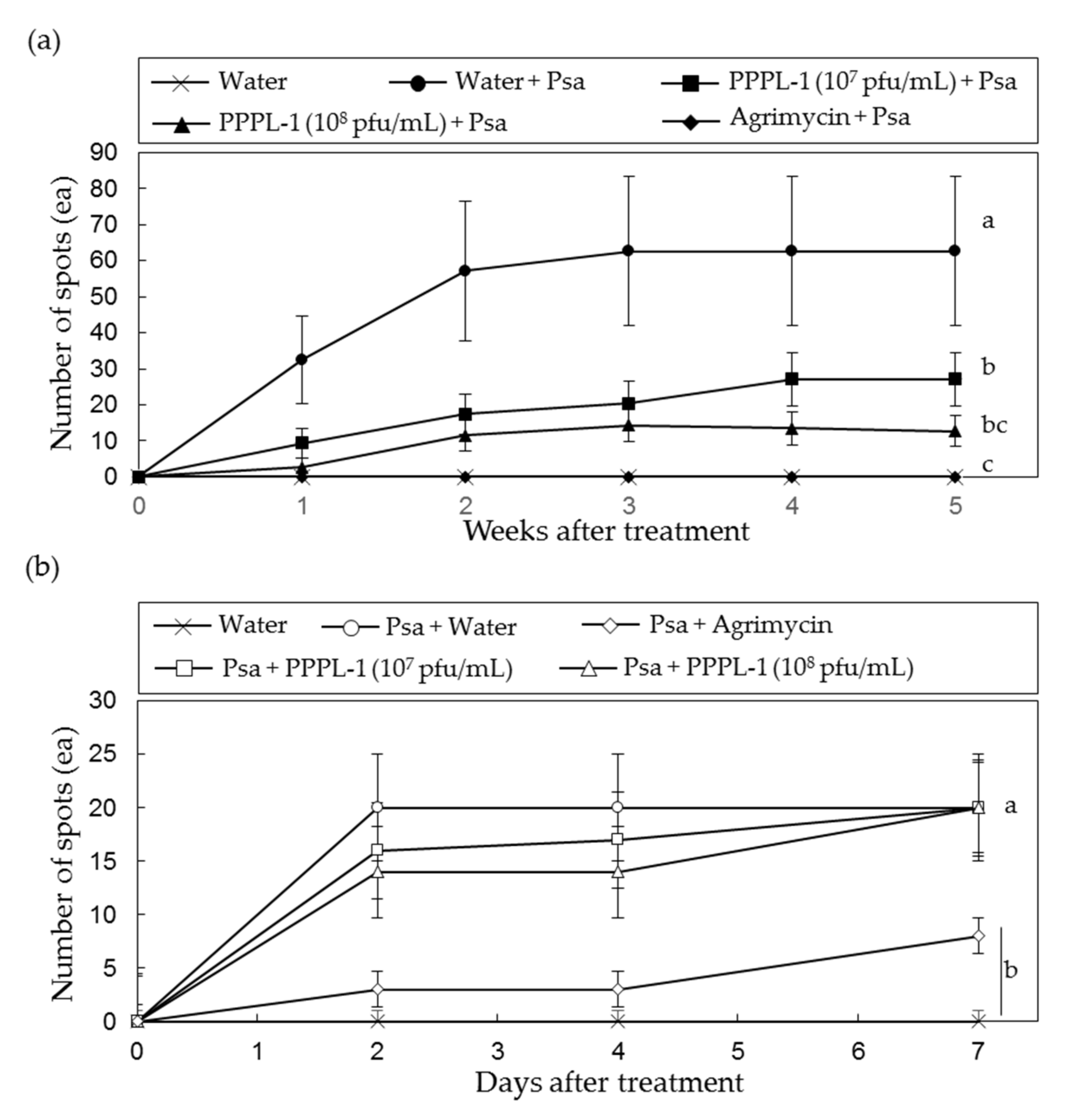
2.1. Control Efficacy of PPPL-1 Phage to Prevent Bacterial Canker in Planta
2.2. Concentration and Treatment Timing of PPPL-1 Phage for Efficient Control of Bacterial Canker in Planta
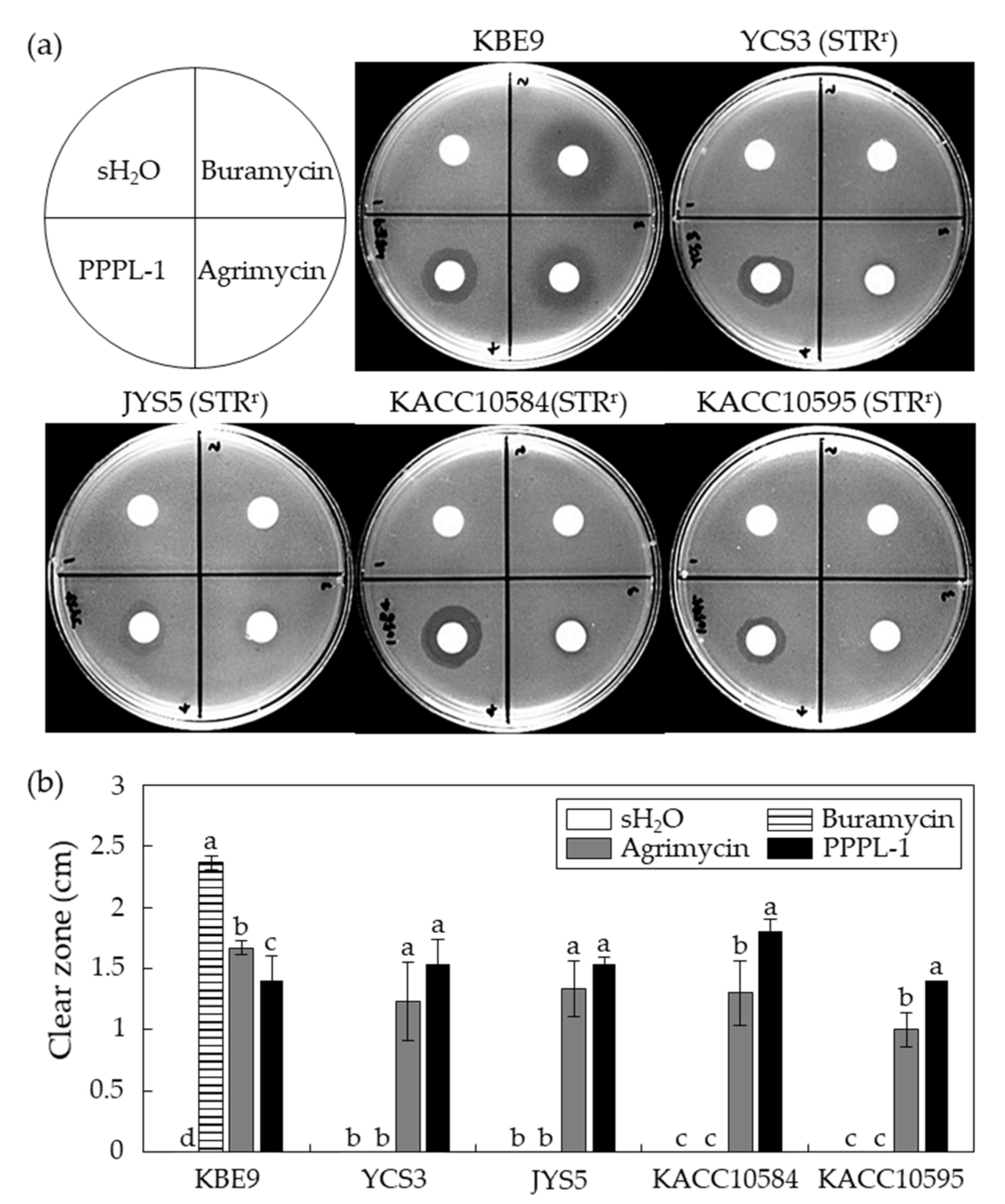
2.3. Antibacterial Effects of PPPL-1 Phage on Streptomycin-Resistant Psa Isolates In Vitro
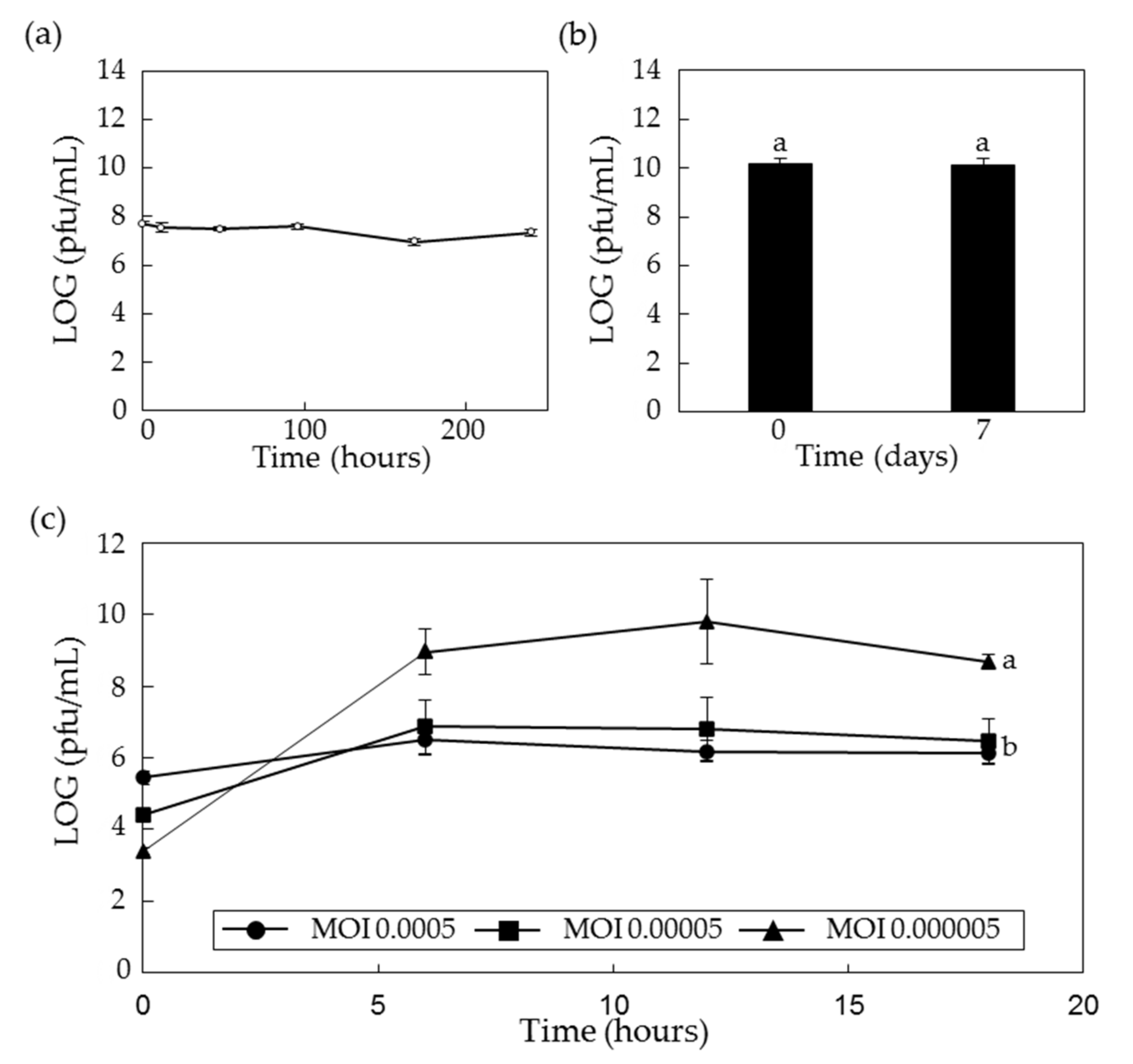
2.4. Stability of PPPL-1 Phage in the Field Soil and Low Temperature
2.5. Optimal Conditions for Scaled-Up Production of PPPL-1 Phage
3. Discussion
4. Materials and Methods
4.1. Growth Conditions of Bacterial Strains
4.2. Phage Lysate Preparation
4.3. Scaled-Up Production and Precipitation of Phages
4.4. Control Efficacy Test of PPPL-1 Phage In Vivo
4.5. Antibacterial Effect of PPPL-1 Phage by Filter Paper Disc Method In Vitro
4.6. Stability Test of PPPL-1 Phage in the Field Soil and Low Temperature
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrante, P.; Scortichini, M. Identification of Pseudomonas syringae pv. actinidiae as causal agent of bacterial canker of yellow kiwifruit (Actinidia chinensis Planchon) in central Italy. J. Phytopathol. 2009, 157, 768–770. [Google Scholar] [CrossRef]
- McCann, H.C.; Li, L.; Liu, Y.; Li, D.; Pan, H.; Zhong, C.; Rikkerink, E.H.; Templeton, M.D.; Straub, C.; Colombi, E. Origin and evolution of the kiwifruit canker pandemic. Genome Biol. Evol. 2017, 9, 932–944. [Google Scholar]
- Wang, F.; Li, J.; Ye, K.; Liu, P.; Gong, H.; Jiang, Q.; Qi, B.; Mo, Q. An in vitro Actinidia bioassay to evaluate the resistance to Pseudomonas syringae pv. actinidiae. Plant Pathol. J. 2019, 35, 372. [Google Scholar] [CrossRef]
- Koh, Y.; Kim, G.; Jung, J.; Lee, Y.; Hur, J. Outbreak of bacterial canker on Hort16A (Actinidia chinensis Planchon) caused by Pseudomonas syringae pv. actinidiae in Korea. N. Z. J. Crop Hortic. Sci. 2010, 38, 275–282. [Google Scholar] [CrossRef]
- Donati, I.; Buriani, G.; Cellini, A.; Mauri, S.; Costa, G.; Spinelli, F. New insights on the bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae). J. Berry Res. 2014, 4, 53–67. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, J.L. The scientific, economic, and social impacts of the New Zealand outbreak of bacterial canker of kiwifruit (Pseudomonas syringae pv. actinidiae). Annu. Rev. Phytopathol. 2017, 55, 377–399. [Google Scholar] [CrossRef]
- Young, J. Pseudomonas syringae pv. actinidiae in New Zealand. J. Plant Pathol. 2012, 94, S1.5–S1.10. [Google Scholar]
- Qin, H.; Gao, X.; Zhao, Z.; Zhu, S.; Li, J.; Huang, L. The prevalence dynamics and rules of bacterial canker of kiwi fruit in Shaanxi. Acta Phytophylacica Sin. 2013, 40, 225–230. [Google Scholar]
- Donati, I.; Cellini, A.; Sangiorgio, D.; Vanneste, J.L.; Scortichini, M.; Balestra, G.M.; Spinelli, F. Pseudomonas syringae pv. actinidiae: Ecology, infection dynamics and disease epidemiology. Microb. Ecol. 2020, 80, 81–102. [Google Scholar] [CrossRef] [PubMed]
- Guroo, I.; Wani, S.; Wani, S.; Ahmad, M.; Mir, S.; Masoodi, F. A review of production and processing of kiwifruit. Int. J. Food Process. Technol. 2017, 8, 699–705. [Google Scholar]
- Takikawa, Y.; Serizawa, S.; Ichikawa, T.; Tsuyumu, S.; Goto, M. Pseudomonas syringae pv. actinidiae pv. nov. the causal bacterium of canker of kiwifruit in Japan. Jpn. J. Phytopathol. 1989, 55, 437–444. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.; Park, S.; Lee, D. Characteristics of bacterial canker of kiwifruit occurring in Korea and its control by trunk injection. Korean J. Plant Pathol. 1996, 12, 324–330. [Google Scholar]
- Balestra, G.; Renzi, M.; Mazzaglia, A. First report of bacterial canker of Actinidia deliciosa caused by Pseudomonas syringae pv. actinidiae in Portugal. New Dis. Rep. 2010, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Balestra, G.; Renzi, M.; Mazzaglia, A. First report of Pseudomonas syringae pv. actinidiae on kiwifruit plants in Spain. N. Dis. Rep. 2011, 24, 10. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, J.; Poliakoff, F.; Audusseau, C.; Cornish, D.; Paillard, S.; Rivoal, C.; Yu, J. First report of Pseudomonas syringae pv. actinidiae, the causal agent of bacterial canker of kiwifruit in France. Plant Dis. 2011, 95, 1311. [Google Scholar] [CrossRef]
- Bastas, K.; Karakaya, A. First report of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae in Turkey. Plant Dis. 2012, 96, 452. [Google Scholar] [CrossRef]
- Dreo, T.; Pirc, M.; Ravnikar, M.; Žežlina, I.; Poliakoff, F.; Rivoal, C.; Nice, F.; Cunty, A. First report of Pseudomonas syringae pv. actinidiae, the causal agent of bacterial canker of kiwifruit in Slovenia. Plant Dis. 2014, 98, 1578. [Google Scholar] [CrossRef]
- Holeva, M.; Glynos, P.; Karafla, C. First report of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae in Greece. Plant Dis. 2015, 99, 723. [Google Scholar] [CrossRef]
- Meparishvili, G.; Gorgiladze, L.; Sikharulidze, Z.; Muradashvili, M.; Koiava, L.; Dumbadze, R.; Jabnidze, N. First report of bacterial canker of kiwifruit caused by Pseudomonas syringae pv. actinidiae in Georgia. Plant Dis. 2016, 100, 517. [Google Scholar] [CrossRef]
- Sawada, H.; Kondo, K.; Nakaune, R. Novel biovar (biovar 6) of Pseudomonas syringae pv. actinidiae causing bacterial canker of kiwifruit (Actinidia deliciosa) in Japan. Jpn. J. Phytopathol. 2016, 82, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Cunty, A.; Poliakoff, F.; Rivoal, C.; Cesbron, S.; Fischer-Le Saux, M.; Lemaire, C.; Jacques, M.; Manceau, C.; Vanneste, J. Characterisation of Pseudomonas syringae pv. actinidiae (Psa) isolated from France and assignment of strains Psa biovar 4 to a de novo pathovar: Pseudomonas syringae pv. actinidifoliorum pv. nov. Plant Pathol. 2015, 64, 582–596. [Google Scholar] [CrossRef]
- Koh, Y.J.; Kim, G.H.; Koh, H.S.; Lee, Y.S.; Kim, S.-C.; Jung, J.S. Occurrence of a new type of Pseudomonas syringae pv. actinidiae strain of bacterial canker on kiwifruit in Korea. Plant Pathol. J. 2012, 28, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Mazzaglia, A.; Studholme, D.J.; Taratufolo, M.C.; Cai, R.; Almeida, N.F.; Goodman, T.; Guttman, D.S.; Vinatzer, B.A.; Balestra, G.M. Pseudomonas syringae pv. actinidiae (PSA) isolates from recent bacterial canker of kiwifruit outbreaks belong to the same genetic lineage. PLoS ONE 2012, 7, e36518. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.I.; Stockwell, P.A.; Black, M.A.; Day, R.C.; Lamont, I.L.; Poulter, R.T. Pseudomonas syringae pv. actinidiae from recent outbreaks of kiwifruit bacterial canker belong to different clones that originated in China. PLoS ONE 2013, 8, e57464. [Google Scholar]
- McCann, H.C.; Rikkerink, E.H.; Bertels, F.; Fiers, M.; Lu, A.; Rees-George, J.; Andersen, M.T.; Gleave, A.P.; Haubold, B.; Wohlers, M.W. Genomic analysis of the kiwifruit pathogen Pseudomonas syringae pv. actinidiae provides insight into the origins of an emergent plant disease. PLoS Pathog. 2013, 9, e1003503. [Google Scholar] [CrossRef]
- Cameron, A.; Sarojini, V. Pseudomonas syringae pv. actinidiae: Chemical control, resistance mechanisms and possible alternatives. Plant Pathol. 2014, 63, 1–11. [Google Scholar] [CrossRef]
- Han, H.S.; Koh, Y.J.; Hur, J.-S.; Jung, J.S. Occurrence of the strA-strB streptomycin resistance genes in Pseudomonas species isolated from kiwifruit plants. J. Microbiol. 2004, 42, 365–368. [Google Scholar]
- Nakajima, M.; Masao, G.; Tadaaki, H. Similarity between copper resistance genes from Pseudomonas syringae pv. actinidiae and P. syringae pv. tomato. J. Gen. Plant Pathol. 2002, 68, 68–74. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, G.H.; Song, Y.-R.; Oh, C.-S.; Koh, Y.J.; Jung, J.S. Streptomycin Resistant Isolates of Pseudomonas syringae pv. actinidiae in Korea. Res. Plant Dis. 2020, 26, 44–47. [Google Scholar] [CrossRef]
- Jones, J.B.; Jackson, L.; Balogh, B.; Obradovic, A.; Iriarte, F.; Momol, M. Bacteriophages for plant disease control. Annu. Rev. Phytopathol. 2007, 45, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Jassim, S.A.; Limoges, R.G. Natural solution to antibiotic resistance: Bacteriophages ‘The Living Drugs’. World J. Microbiol. Biotechnol. 2014, 30, 2153–2170. [Google Scholar] [CrossRef] [Green Version]
- Brüssow, H. What is needed for phage therapy to become a reality in Western medicine? Virology 2012, 434, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Vu, N.T.; Oh, C.-S. Bacteriophage Usage for Bacterial Disease Management and Diagnosis in Plants. Plant Pathol. J. 2020, 36, 204–217. [Google Scholar] [CrossRef]
- Yin, Y.; Ni, P.e.; Deng, B.; Wang, S.; Xu, W.; Wang, D. Isolation and characterisation of phages against Pseudomonas syringae pv. actinidiae. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 199–208. [Google Scholar] [CrossRef]
- Di Lallo, G.; Evangelisti, M.; Mancuso, F.; Ferrante, P.; Marcelletti, S.; Tinari, A.; Superti, F.; Migliore, L.; D’Addabbo, P.; Frezza, D. Isolation and partial characterization of bacteriophages infecting Pseudomonas syringae pv. actinidiae, causal agent of kiwifruit bacterial canker. J. Basic Microbiol. 2014, 54, 1210–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frampton, R.A.; Taylor, C.; Moreno, A.V.H.; Visnovsky, S.B.; Petty, N.K.; Pitman, A.R.; Fineran, P.C. Identification of bacteriophages for biocontrol of the kiwifruit canker phytopathogen Pseudomonas syringae pv. actinidiae. Appl. Environ. Microbiol. 2014, 80, 2216–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.-G.; Lim, J.-A.; Song, Y.-R.; Heu, S.; Kim, G.H.; Koh, Y.J.; Oh, C.-S. Isolation and characterization of bacteriophages against Pseudomonas syringae pv. actinidiae causing bacterial canker disease in kiwifruit. J. Microbiol. Biotechnol. 2016, 26, 385–393. [Google Scholar]
- Pinheiro, L.A.; Pereira, C.; Barreal, M.E.; Gallego, P.P.; Balcão, V.M.; Almeida, A. Use of phage ϕ6 to inactivate Pseudomonas syringae pv. actinidiae in kiwifruit plants: In vitro and ex vivo experiments. Appl. Microbiol. Biotechnol. 2020, 104, 1319–1330. [Google Scholar] [CrossRef]
- Park, J.; Lim, J.-A.; Yu, J.-G.; Oh, C.-S. Genomic features and lytic activity of the bacteriophage PPPL-1 effective against Pseudomonas syringae pv. actinidiae, a cause of bacterial canker in kiwifruit. J. Microbiol. Biotechnol. 2018, 28, 1542–1546. [Google Scholar] [CrossRef]
- Han, H.-S.; Nam, H.-Y.; Koh, Y.-J.; Hur, J.-S.; Jung, J.-S. Molecular bases of high-level streptomycin resistance in Pseudomonas marginalis and Pseudomonas syringae pv. actinidiae. J. Microbiol. 2003, 41, 16–21. [Google Scholar]
- Scortichini, M.; Marcelletti, S.; Ferrante, P.; Petriccione, M.; Firrao, G. Pseudomonas syringae pv. actinidiae: A re-emerging, multi-faceted, pandemic pathogen. Mol. Plant Pathol. 2012, 13, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Flores, O.; Retamales, J.; Núñez, M.; León, M.; Salinas, P.; Besoain, X.; Yañez, C.; Bastías, R. Characterization of Bacteriophages against Pseudomonas syringae pv. actinidiae with Potential Use as Natural Antimicrobials in Kiwifruit Plants. Microorganisms 2020, 8, 974. [Google Scholar] [CrossRef]
- Preti, M.; Scannavini, M.; Franceschelli, F.; Cavazza, F.; Antoniacci, L.; Rossi, R.; Bugiani, R.; Tommasini, M.; Collina, M. Field efficacy of some products against the bacterial canker of kiwifruit in Emilia-Romagna Region, Italy. Acta Hortic. 2015, 1243, 39–48. [Google Scholar] [CrossRef]
- Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.A.; Momol, M.T.; Jones, J.B.; Vallad, G.E. Soil-based systemic delivery and phyllosphere in vivo propagation of bacteriophages: Two possible strategies for improving bacteriophage persistence for plant disease control. Bacteriophage 2012, 2, e23530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombouts, S.; Volckaert, A.; Venneman, S.; Declercq, B.; Vandenheuvel, D.; Allonsius, C.N.; Van Malderghem, C.; Jang, H.B.; Briers, Y.; Noben, J.P. Characterization of novel bacteriophages for biocontrol of bacterial blight in leek caused by Pseudomonas syringae pv. porri. Front. Microbiol. 2016, 7, 279. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wei, Z.; Yang, K.; Wang, J.; Jousset, A.; Xu, Y.; Shen, Q.; Friman, V.-P. Phage combination therapies for bacterial wilt disease in tomato. Nat. Biotechnol. 2019, 37, 1513–1520. [Google Scholar] [CrossRef]
- Lang, J.M.; Gent, D.H.; Schwartz, H.F. Management of Xanthomonas leaf blight of onion with bacteriophages and a plant activator. Plant Dis. 2007, 91, 871–878. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. In Bacteriophages; Springer’s Humana Press: Totowa, NJ, USA, 2009; pp. 69–76. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-R.; Vu, N.T.; Park, J.; Hwang, I.S.; Jeong, H.-J.; Cho, Y.-S.; Oh, C.-S. Phage PPPL-1, A New Biological Agent to Control Bacterial Canker Caused by Pseudomonas syringae pv. actinidiae in Kiwifruit. Antibiotics 2021, 10, 554. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050554
Song Y-R, Vu NT, Park J, Hwang IS, Jeong H-J, Cho Y-S, Oh C-S. Phage PPPL-1, A New Biological Agent to Control Bacterial Canker Caused by Pseudomonas syringae pv. actinidiae in Kiwifruit. Antibiotics. 2021; 10(5):554. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050554
Chicago/Turabian StyleSong, Yu-Rim, Nguyen Trung Vu, Jungkum Park, In Sun Hwang, Hyeon-Ju Jeong, Youn-Sup Cho, and Chang-Sik Oh. 2021. "Phage PPPL-1, A New Biological Agent to Control Bacterial Canker Caused by Pseudomonas syringae pv. actinidiae in Kiwifruit" Antibiotics 10, no. 5: 554. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050554





