Quantification and Trends of Antimicrobial Use in Commercial Broiler Chicken Production in Pakistan
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Selection Criteria
2.3. Data Collection
2.4. Antimicrobial Use Calculations
2.4.1. Antimicrobial Active Ingredient (AAI)
2.4.2. Milligrams of AAI Used per Population Correction Unit (mg/PCU)
2.4.3. Milligrams of AAI Used per Final Flock Weight (mg/FFW)
2.5. Calculations for WHO Critically Important Antimicrobial Classes
3. Results
3.1. Response to Antimicrobial Use Data Collection
3.2. Population Size and Housing
3.3. Quantitaive Antimicrobial Use in Broiler Chicken
3.4. Seasonal Variations in Antimicrobial Use in Broiler Chicken
3.5. Use of WHO-CIA in Broiler Chicken
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Global Action Plan on Antimicrobial Resistance. Available online: https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1 (accessed on 5 May 2021).
- FAO. The FAO Action Plan on Antimicrobial Resistance 2016–2020. Available online: http://www.fao.org/3/a-i5996e.pdf (accessed on 5 May 2021).
- OIE. The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. Available online: https://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf (accessed on 5 May 2021).
- GOP. National AMR Action Plan for Pakistan. Available online: https://www.nih.org.pk/wp-content/uploads/2018/08/AMR-National-Action-Plan-Pakistan.pdf (accessed on 5 May 2021).
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Schar, D.; Sommanustweechai, A.; Laxminarayan, R.; Tangcharoensathien, V. Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med. 2018, 15, e1002521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsin, M.; Van Boeckel, T.P.; Saleemi, M.K.; Umair, M.; Naseem, M.N.; He, C.; Khan, A.; Laxminarayan, R. Excessive use of medically important antimicrobials in food animals in Pakistan: A five-year surveillance survey. Glob. Health Action 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Working Party on Agricultural Policies and Markets Antibiotic Use and Antibiotic Resistance in Food Producing Animals in China. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=TAD/CA/APM/WP(2018)19/FINAL&docLanguage=En (accessed on 5 May 2021).
- ESVAC. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 5 May 2021).
- WHO. Critically Important Antimicrobials for Human Medicine, 6th Revision. Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf?ua=1 (accessed on 5 May 2021).
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Borck Høg, B.; Dalhoff Andersen, V.; Chauvin, C.; et al. Monitoring of Farm-Level Antimicrobial Use to Guide Stewardship: Overview of Existing Systems and Analysis of Key Components and Processes. Front. Vet. Sci. 2020, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Gow, S.P.; Léger, D.F.; Deckert, A.E.; Carson, C.A.; Bosman, A.L.; Kadykalo, S.; Reid-Smith, R.J. Antimicrobial Use Indices—The Value of Reporting Antimicrobial Use in Multiple Ways Using Data From Canadian Broiler Chicken and Turkey Farms. Front. Vet. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Agunos, A.; Gow, S.P.; Léger, D.F.; Carson, C.A.; Deckert, A.E.; Bosman, A.L.; Loest, D.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial Use and Antimicrobial Resistance Indicators—Integration of Farm-Level Surveillance Data From Broiler Chickens and Turkeys in British Columbia, Canada. Front. Vet. Sci. 2019, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Rahmatallah, N.; El Rhaffouli, H.; Lahlou Amine, I.; Sekhsokh, Y.; Fassi Fihri, O.; El Houadfi, M. Consumption of antibacterial molecules in broiler production in Morocco. Vet. Med. Sci. 2018, 4, 80–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NethMap. Consumption of Antimicrobial Agents and Antimicrobial Resistance among Medically Important Bacteria in The Netherlands in 2019/MARAN 2020: Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2019. Available online: https://www.rivm.nl/bibliotheek/rapporten/2020-0065.pdf (accessed on 5 May 2021).
- ANSES. Sales Survey of Veterinary Medicinal Products Containing Antimicrobials in France in 2019. Available online: https://www.anses.fr/en/system/files/ANMV-Ra-Antibiotiques2019EN.pdf (accessed on 5 May 2021).
- BELVETSAC. Belgian Veterinary Surveillance on Antimicrobial Consumption Report 2019. Available online: https://belvetsac.ugent.be/BelvetSac_report_2019.pdf (accessed on 5 May 2021).
- DANMAP. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria From Food Animals, Food and Humans in Denmark. Available online: https://www.danmap.org/-/media/Sites/danmap/Downloads/Reports/2019/DANMAP_2019.ashx?la=da&hash=AA1939EB449203EF0684440AC1477FFCE2156BA5 (accessed on 5 May 2021).
- Swedres-Svarm. Sales of Antibiotics and Occurrence of Resistance in Sweden. Available online: https://www.folkhalsomyndigheten.se/contentassets/fb80663bc7c94d678be785e3360917d1/swedres-svarm-2019.pdf (accessed on 5 May 2021).
- UK-VARSS. UK Veterinary Antibiotic Resistance and Sales Surveillance Report (UK-VARSS 2018). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/909987/_1587146-v23-VARSS_2018_Report__2019_-accessible.pdf (accessed on 5 May 2021).
- PMD. Monthly Weather Reports National Weather Forecasting Centre, Islamabad. Available online: https://nwfc.pmd.gov.pk/new/monthly-reports.php (accessed on 5 May 2021).
- Miles, D.M.; Rowe, D.E.; Owens, P.R. Winter broiler litter gases and nitrogen compounds: Temporal and spatial trends. Atmos. Environ. 2008, 42, 3351–3363. [Google Scholar] [CrossRef]
- Shoaib, M.; Riaz, A.; ul Hassan, M.; Yousaf, A.; Rehman, S.U.; Zafar, M.A.; Kamran, M.; Amir, R.M.; Malik, A.M. Sero-prevalence and associated risk factors of Mycoplasma gallisepticum, Mycoplasma synoviae and Salmonella pullorum/gallinarium in poultry. Pak. Vet. J. 2020, 40, 253–256. [Google Scholar] [CrossRef]
- Hussnain, F.; Mahmud, A.; Mehmood, S.; Jaspal, M.H. Effect of broiler crating density and transportation distance on preslaughter losses and physiological response during the winter season in Punjab, Pakistan. Rev. Bras. Cienc. Avic. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Agunos, A.; Léger, D.F.; Carson, C.A.; Gow, S.P.; Bosman, A.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial use surveillance in broiler chicken flocks in Canada, 2013-2015. PLoS ONE 2017, 12, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Persoons, D.; Dewulf, J.; Smet, A.; Herman, L.; Heyndrickx, M.; Martel, A.; Catry, B.; Butaye, P.; Haesebrouck, F. Antimicrobial use in Belgian broiler production. Prev. Vet. Med. 2012, 105, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Wongsuvan, G.; Wuthiekanun, V.; Hinjoy, S.; Day, N.P.; Limmathurotsakul, D. Antibiotic use in poultry: A survey of eight farms in Thailand. Bull. World Health Organ. 2018, 96, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Joosten, P.; Sarrazin, S.; Van Gompel, L.; Luiken, R.E.C.; Mevius, D.J.; Wagenaar, J.A.; Heederik, D.J.J.; Dewulf, J.; Graveland, H.; Schmitt, H.; et al. Quantitative and qualitative analysis of antimicrobial usage at farm and flock level on 181 broiler farms in nine European countries. J. Antimicrob. Chemother. 2019, 74, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Van Cuong, N.; Phu, D.H.; Van, N.T.B.; Dinh Truong, B.; Kiet, B.T.; Hien, B.V.; Thu, H.T.V.; Choisy, M.; Padungtod, P.; Thwaites, G.; et al. High-Resolution Monitoring of Antimicrobial Consumption in Vietnamese Small-Scale Chicken Farms Highlights Discrepancies Between Study Metrics. Front. Vet. Sci. 2019, 6, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Punjab | KPK | ||||
|---|---|---|---|---|---|
| Summer | |||||
| Farm ID | Location (District) | No. of Birds | Farm ID | Location (District) | No. of Birds |
| PP-1 | Faisalabad | 26,500 | KP-1 | Mardan | 56,000 |
| PP-2 | Sargodha | 30,000 | KP-2 | Mardan | 52,000 |
| PP-3 | Sargodha | 30,000 | KP-3 | Peshawar | 108,000 |
| PP-4 | Faisalabad | 19,900 | KP-4 | Lower Dir | 6000 |
| PP-5 | Lahore | 64,600 | KP-5 | Lower Dir | 3000 |
| PP-6 | Sheikhupura | 81,600 | -- | -- | -- |
| Winter | |||||
| PP-1 | Faisalabad | 24,000 | KP-1 | Mardan | 58,000 |
| PP-4 | Faisalabad | 15,000 | KP-2 | Mardan | 56,000 |
| PP-7 | Sargodha | 28,500 | KP-6 | Lower Dir | 6800 |
| PP-8 | Sargodha | 29,000 | KP-7 | Lower Dir | 7500 |
| PP-9 | Faisalabad | 26,000 | KP-8 | Swabi | 3500 |
| PP-10 | Kasur | 30,600 | KP-9 | Mansehra | 3000 |
| Antimicrobial Class | Antimicrobial | AAI | mg/PCU | mg/FFW | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | KPK | Pb | KPK | Pb | KPK | AAI | mg/PCU | mg/FFW | ||
| Antiviral | Amantadine | 2.21 | 0 | 22.11 | 0 | 10.21 | 0 | 2.21 | 22.11 | 10.21 |
| Aminopenicillins | Amoxicillin | 6.69 | 9.52 | 78.59 | 44.18 | 36.35 | 21.91 | 16.2 | 53.92 | 26.2 |
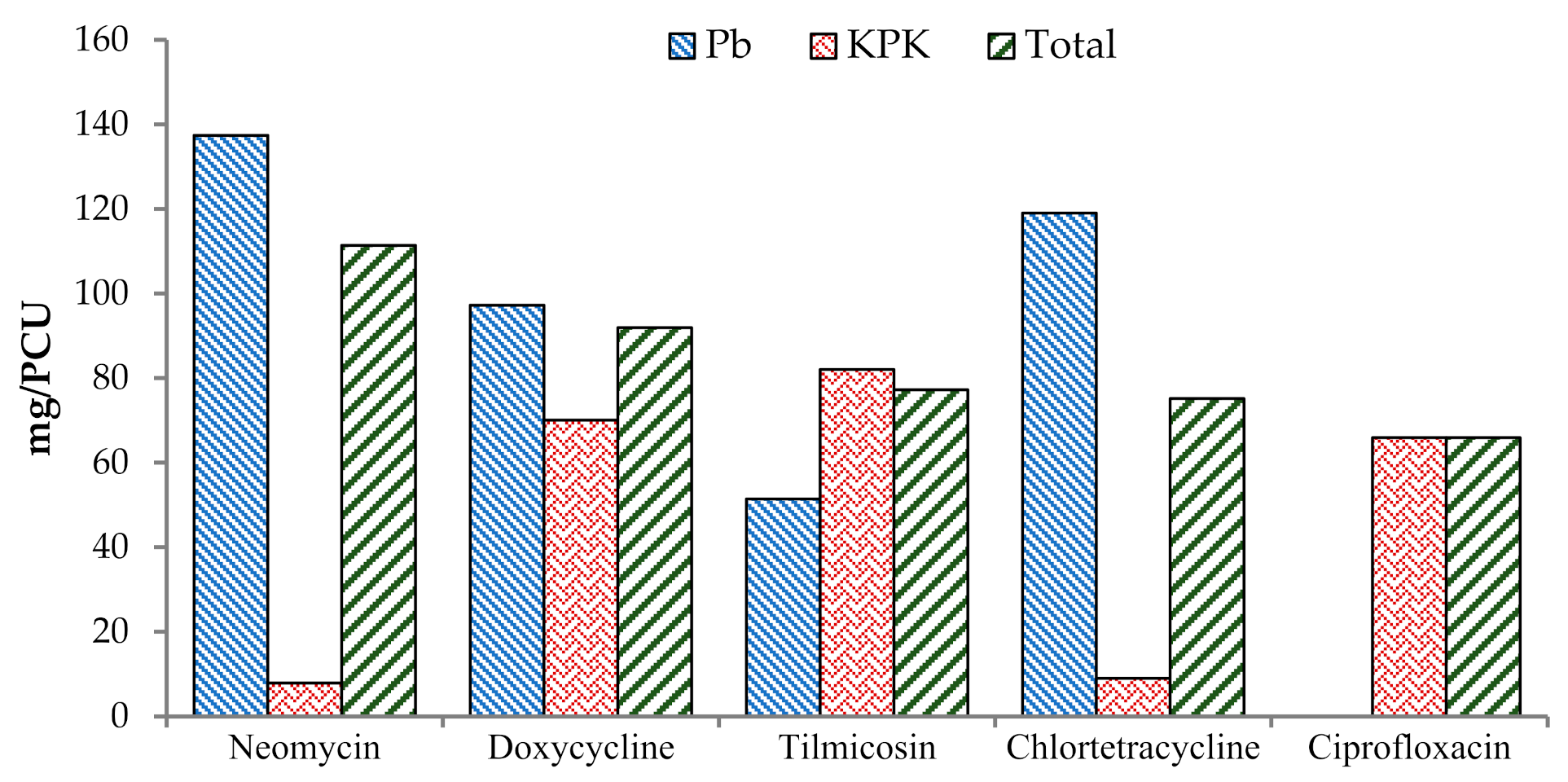
| Tetracyclines | Chlortetracycline | 9.89 | 0.5 | 119 | 9.01 | 57.33 | 4.69 | 10.39 | 75.16 | 37.3 |
| Quinolones and fluoroquinolones | Ciprofloxacin | 0 | 3.63 | 0 | 65.9 | 0 | 34.27 | 3.63 | 65.9 | 34.27 |
| Polymyxins | Colistin | 3.89 | 1.75 | 15.65 | 7.91 | 7.33 | 3.94 | 5.64 | 12.01 | 5.78 |
| Tetracyclines | Doxycycline | 24.16 | 4.27 | 97.26 | 70.08 | 45.54 | 36.8 | 28.44 | 91.91 | 43.97 |
| Quinolones and fluoroquinolones | Enrofloxacin | 7.18 | 0.93 | 31.37 | 8.56 | 14.7 | 4.06 | 8.11 | 24 | 11.29 |
| Amphenicols | Florfenicol | 1.78 | 0.76 | 28.02 | 12.52 | 13.64 | 6.58 | 2.54 | 20.43 | 10.32 |
| Phosphonic acid derivatives | Fosfomycin | 0 | 0.07 | 0 | 23.93 | 0 | 14.53 | 0.07 | 23.93 | 14.53 |
| Nitrofurans derivatives | Furaltadone | 0.96 | 0 | 49.22 | 0 | 22.93 | 0 | 0.96 | 49.22 | 22.93 |
| Lincosamides | Lincomycin | 2.2 | 0.03 | 37.28 | 10.46 | 18.35 | 6.35 | 2.23 | 36 | 17.89 |
| Aminoglycosides | Neomycin | 30.09 | 0.43 | 137.43 | 7.89 | 63.89 | 4.1 | 30.52 | 111.39 | 52.91 |
| Quinolones and fluoroquinolones | Norfloxacin | 1.24 | 1.42 | 63.15 | 24.85 | 29.42 | 13.06 | 2.65 | 34.64 | 17.62 |
| Tetracyclines | Oxytetracycline | 3.3 | 0 | 59.38 | 0 | 26.55 | 0 | 3.3 | 59.38 | 26.55 |
| Aminoglycosides | Streptomycin | 0 | 0.03 | 0 | 10.46 | 0 | 6.35 | 0.03 | 10.46 | 6.35 |
| Sulfonamides | Sulfadiazine | 4.28 | 0 | 53.36 | 0 | 24.6 | 0 | 4.28 | 53.36 | 24.6 |
| Macrolides and ketolides | Tilmicosin | 1.52 | 12.91 | 51.43 | 82.06 | 25.44 | 39.91 | 14.43 | 77.22 | 37.65 |
| Dihydrofolate reductase inhibitors | Trimethoprim | 0.86 | 0 | 10.67 | 0 | 4.92 | 0 | 0.86 | 10.67 | 4.92 |
| Macrolides and ketolides | Tylosin | 12.08 | 2.15 | 48.63 | 33.7 | 22.77 | 17.81 | 14.24 | 45.58 | 21.85 |
| Total | 112.32 | 38.42 | 452.11 | 173.6 | 211.69 | 86.41 | 150.74 | 320.9 | 154.58 | |
| Antimicrobial Class | Antimicrobial | AAI | mg/PCU | mg/FFW | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pb | KPK | Pb | KPK | Pb | KPK | AAI | mg/PCU | mg/FFW | ||
| Antiviral | Amantadine | 0 | 0.06 | 0 | 17.65 | 0 | 9.83 | 0.06 | 17.65 | 9.83 |
| Aminopenicillins | Amoxicillin | 15.2 | 11.74 | 142.01 | 92.3 | 58.79 | 51.78 | 26.94 | 115.04 | 55.53 |
| Tetracyclines | Chlortetracycline | 1.4 | 0 | 46.4 | 0 | 19.95 | 0 | 1.4 | 46.4 | 19.95 |
| Polymyxins | Colistin | 3.24 | 15.84 | 21.57 | 118.65 | 9.19 | 66.51 | 19.08 | 67.22 | 32.28 |
| Tetracyclines | Doxycycline | 12.2 | 38.7 | 89.98 | 314.52 | 38.28 | 176.51 | 50.9 | 196.81 | 94.61 |
| Quinolones and fluoroquinolones | Enrofloxacin | 4 | 4.18 | 37.95 | 60.98 | 16.09 | 38.7 | 8.18 | 47.02 | 22.94 |
| Amphenicols | Florfenicol | 3.54 | 0.23 | 42.49 | 30.69 | 17.03 | 17.04 | 3.77 | 41.5 | 17.03 |
| Phosphonic acid derivatives | Fosfomycin | 0 | 0.4 | 0 | 27.99 | 0 | 15.96 | 0.4 | 27.99 | 15.96 |
| Nitrofurans derivatives | Furaltadone | 1.05 | 0 | 34.8 | 0 | 14.96 | 0 | 1.05 | 34.8 | 14.96 |
| Lincosamides | Lincomycin | 4.93 | 3.78 | 46.75 | 68.3 | 19.83 | 44.55 | 8.71 | 54.16 | 26.12 |
| Aminoglycosides | Neomycin | 18.54 | 0 | 136.74 | 0 | 58.17 | 0 | 18.54 | 136.74 | 58.17 |
| Quinolones and fluoroquinolones | Norfloxacin | 2 | 4.3 | 135.36 | 77.62 | 58.36 | 50.62 | 6.3 | 89.78 | 52.85 |
| Tetracyclines | Oxytetracycline | 6.6 | 0 | 85.87 | 0 | 35.04 | 0 | 6.6 | 85.87 | 35.04 |
| Sulfonamides | Sulfamethazine | 0 | 2.5 | 0 | 45.13 | 0 | 29.43 | 2.5 | 45.13 | 29.43 |
| Sulfonamides | Sulfamethoxypyridazine | 0 | 3.75 | 0 | 67.69 | 0 | 44.15 | 3.75 | 67.69 | 44.15 |
| Aminocyclitols | Spectinomycin | 0.53 | 3.78 | 19.2 | 68.3 | 6.55 | 44.55 | 4.31 | 51.99 | 26.04 |
| Macrolides and ketolides | Tilmicosin | 1.5 | 4.75 | 52.54 | 42.09 | 24.92 | 23.56 | 6.25 | 44.2 | 23.87 |
| Dihydrofolate reductase inhibitors | Trimethoprim | 0 | 1.25 | 0 | 22.56 | 0 | 14.72 | 1.25 | 22.56 | 14.72 |
| Macrolides and ketolides | Tylosin | 6.1 | 21.76 | 44.99 | 163 | 19.14 | 91.37 | 27.86 | 103.54 | 50.03 |
| Total | 80.83 | 117.02 | 936.65 | 1217.47 | 396.3 | 719.28 | 197.85 | 697.01 | 334.68 | |
| WHO-CIA Classes | Antimicrobial Class | Antimicrobial | Summer | Winter | mg/PCU % Diff * | Punjab | KPK | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAI | mg/FFW | mg/PCU | AAI | mg/FFW | mg/PCU | AAI | mg/FFW | mg/PCU | AAI | mg/FFW | mg/PCU | AAI | mg/FFW | mg/PCU | ||||
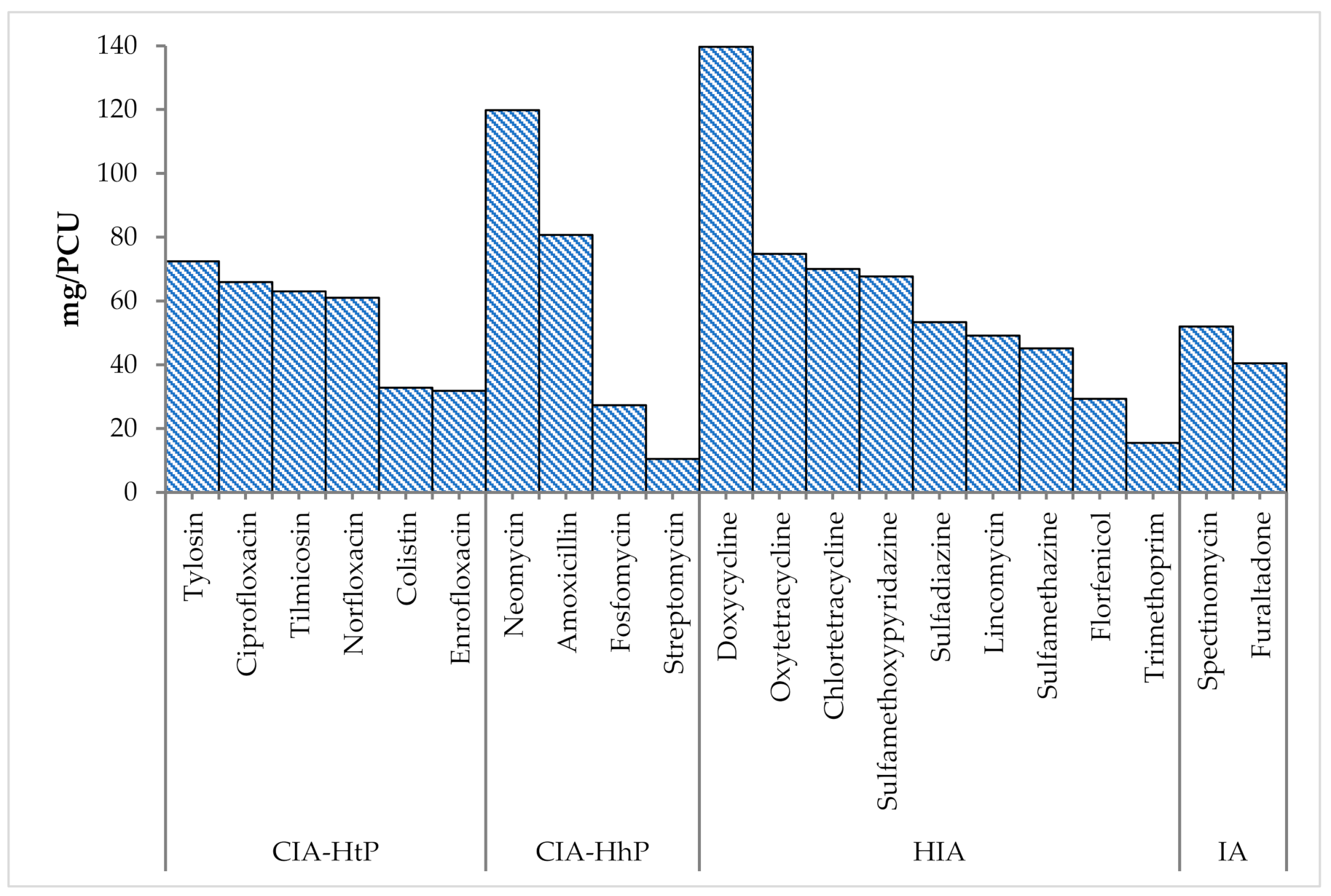
| - | Antiviral | Amantadine | 2.21 | 10.21 | 22.11 | 0.06 | 9.83 | 17.65 | −20.17 | 2.21 | 10.21 | 22.11 | 0.06 | 9.83 | 17.65 | 2.27 | 10.2 | 21.96 |
| CIA-HhP | Aminopenicillins | Amoxicillin | 16.2 | 26.2 | 53.92 | 26.94 | 55.53 | 115.04 | 113.35 | 21.89 | 49.46 | 113.92 | 21.26 | 32.16 | 62.05 | 43.14 | 39.1 | 80.69 |
| HIA | Tetracyclines | Chlortetracycline | 10.39 | 37.3 | 75.16 | 1.4 | 19.95 | 46.4 | −38.27 | 11.29 | 46.52 | 99.66 | 0.5 | 4.69 | 9.01 | 11.79 | 33.81 | 70.01 |
| CIA-HtP | Quinolones and fluoroquinolones | Ciprofloxacin | 3.63 | 34.27 | 65.9 | 0 | 0 | 0 | −100 | 0 | 0 | 0 | 3.63 | 34.27 | 65.9 | 3.63 | 34.27 | 65.9 |
| CIA-HtP | Polymyxins | Colistin | 5.64 | 5.78 | 12.01 | 19.08 | 32.28 | 67.22 | 459.7 | 7.13 | 8.07 | 17.88 | 17.59 | 25.77 | 49.58 | 24.72 | 15.78 | 32.8 |
| HIA | Tetracyclines | Doxycycline | 28.44 | 43.97 | 91.91 | 50.9 | 94.61 | 196.81 | 114.13 | 36.36 | 42.82 | 94.69 | 42.97 | 128.14 | 233.52 | 79.34 | 66.97 | 139.67 |
| CIA-HtP | Quinolones and fluoroquinolones | Enrofloxacin | 8.11 | 11.29 | 24 | 8.18 | 22.94 | 47.02 | 95.92 | 11.18 | 15.17 | 33.44 | 5.11 | 15.13 | 28.78 | 16.29 | 15.15 | 31.82 |
| HIA | Amphenicols | Florfenicol | 2.54 | 10.32 | 20.43 | 3.77 | 17.03 | 41.5 | 103.13 | 5.32 | 15.72 | 36.22 | 0.99 | 7.67 | 14.51 | 6.31 | 13.49 | 29.32 |
| CIA-HhP | Phosphonic acid derivatives | Fosfomycin | 0.07 | 14.53 | 23.93 | 0.4 | 15.96 | 27.99 | 16.97 | 0 | 0 | 0 | 0.47 | 15.73 | 27.3 | 0.47 | 15.73 | 27.3 |
| IA | Nitrofurans derivatives | Furaltadone | 0.96 | 22.93 | 49.22 | 1.05 | 14.96 | 34.8 | −29.3 | 2.01 | 17.95 | 40.47 | 0 | 0 | 0 | 2.01 | 17.95 | 40.47 |
| HIA | Lincosamides | Lincomycin | 2.23 | 17.89 | 36 | 8.71 | 26.12 | 54.16 | 50.44 | 7.13 | 19.35 | 43.36 | 3.81 | 42.44 | 65.31 | 10.94 | 23.88 | 49.11 |
| CIA-HhP | Aminoglycosides | Neomycin | 30.52 | 52.91 | 111.39 | 18.54 | 58.17 | 136.74 | 22.76 | 48.63 | 61.58 | 137.16 | 0.43 | 4.1 | 7.89 | 49.06 | 54.78 | 119.78 |
| CIA-HtP | Quinolones and fluoroquinolones | Norfloxacin | 2.65 | 17.62 | 34.64 | 6.3 | 52.85 | 89.78 | 159.18 | 3.24 | 42.42 | 94.21 | 5.72 | 29.55 | 50.85 | 8.95 | 33.19 | 61 |
| HIA | Tetracyclines | Oxytetracycline | 3.3 | 26.55 | 59.38 | 6.6 | 35.04 | 85.87 | 44.61 | 9.9 | 31.66 | 74.75 | 0 | 0 | 0 | 9.9 | 31.66 | 74.75 |
| IA | Aminocyclitols | Spectinomycin | 0 | 0 | 0 | 4.31 | 26.04 | 51.99 | NUS | 0.53 | 6.58 | 19.27 | 3.78 | 44.5 | 68.23 | 4.31 | 26.04 | 51.99 |
| CIA-HhP | Aminoglycosides | Streptomycin | 0.03 | 6.35 | 10.46 | 0 | 0 | 0 | −100 | 0 | 0 | 0 | 0.03 | 6.35 | 10.46 | 0.03 | 6.35 | 10.46 |
| HIA | Sulfonamides | Sulfadiazine | 4.28 | 24.6 | 53.36 | 0 | 0 | 0 | −100 | 4.28 | 24.6 | 53.36 | 0 | 0 | 0 | 4.28 | 24.6 | 53.36 |
| HIA | Sulfonamides | Sulfamethazine | 0 | 0 | 0 | 2.5 | 29.43 | 45.13 | NUS | 0 | 0 | 0 | 2.5 | 29.43 | 45.13 | 2.5 | 29.43 | 45.13 |
| HIA | Sulfonamides | Sulfamethoxypyridazine | 0 | 0 | 0 | 3.75 | 44.15 | 67.69 | NUS | 0 | 0 | 0 | 3.75 | 44.15 | 67.69 | 3.75 | 44.15 | 67.69 |
| CIA-HtP | Macrolides and ketolides | Tilmicosin | 14.43 | 37.65 | 77.22 | 6.25 | 23.87 | 44.2 | −42.76 | 3.02 | 25.18 | 51.98 | 17.66 | 33.63 | 65.37 | 20.68 | 32.06 | 63 |
| HIA | Dihydrofolate reductase inhibitors | Trimethoprim | 0.86 | 4.92 | 10.67 | 1.25 | 14.72 | 22.56 | 111.43 | 0.86 | 4.92 | 10.67 | 1.25 | 14.72 | 22.56 | 2.11 | 8.13 | 15.53 |
| CIA-HtP | Macrolides and ketolides | Tylosin | 14.24 | 21.85 | 45.58 | 27.86 | 50.03 | 103.54 | 127.16 | 18.18 | 21.41 | 47.35 | 23.91 | 66.59 | 121.13 | 42.1 | 34.83 | 72.4 |
| Total | 150.74 | 154.58 | 320.9 | 197.85 | 334.68 | 697.01 | 117.2 | 193.15 | 218.6 | 484.33 | 155.44 | 227.67 | 438.1 | 348.59 | 222.55 | 462.57 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umair, M.; Tahir, M.F.; Ullah, R.W.; Ali, J.; Siddique, N.; Rasheed, A.; Akram, M.; Zaheer, M.U.; Mohsin, M. Quantification and Trends of Antimicrobial Use in Commercial Broiler Chicken Production in Pakistan. Antibiotics 2021, 10, 598. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050598
Umair M, Tahir MF, Ullah RW, Ali J, Siddique N, Rasheed A, Akram M, Zaheer MU, Mohsin M. Quantification and Trends of Antimicrobial Use in Commercial Broiler Chicken Production in Pakistan. Antibiotics. 2021; 10(5):598. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050598
Chicago/Turabian StyleUmair, Muhammad, Muhammad Farooq Tahir, Riasat Wasee Ullah, Jabir Ali, Naila Siddique, Ayesha Rasheed, Muhammad Akram, Muhammad Usman Zaheer, and Mashkoor Mohsin. 2021. "Quantification and Trends of Antimicrobial Use in Commercial Broiler Chicken Production in Pakistan" Antibiotics 10, no. 5: 598. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10050598








