Prevalence and Impact of Biofilms on Bloodstream and Urinary Tract Infections: A Systematic Review and Meta-Analysis
Abstract
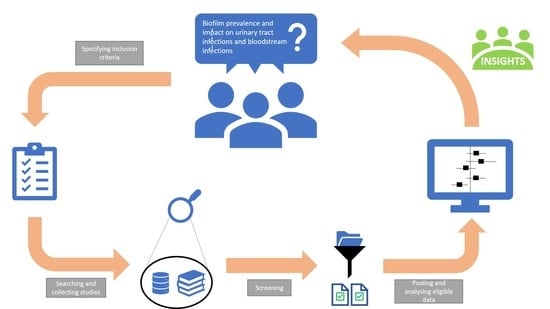
:1. Introduction
2. Results and Discussion
2.1. Bloodstream Infections
2.1.1. Literature Search and Study Selection
2.1.2. Study Characteristics
2.1.3. BFP Unrelated Prevalence: Single-Armed Meta-Analysis
2.1.4. BFP Prevalence Related to Resistance: Two-Armed Meta-Analysis
2.1.5. BFP Prevalence Related to Persistence: Two-Armed Meta-Analysis
2.1.6. BFP Prevalence Related to Mortality: Two-Armed Meta-Analysis
2.2. Urinary Tract Infections
2.2.1. Literature Search and Study Selection
2.2.2. Study Characteristics
2.2.3. BFP Unrelated Prevalence: Single-Armed Meta-Analysis
2.2.4. BFP Prevalence Related to Resistance: Two-Armed Meta-Analysis
2.2.5. BFP Prevalence Related to CAUTI: Two-Armed Meta-Analysis
3. Materials and Methods
3.1. Literature Search
3.2. Study Selection
3.3. Data Extraction
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef] [Green Version]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID) Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Waters, V.; Ratjen, F. Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis. Cochrane Database Syst. Rev. 2017, 10, CD009528. [Google Scholar] [CrossRef]
- Franco-Duarte, R.; Černáková, L.; Kadam, S.; Kaushik, K.S.; Salehi, B.; Bevilacqua, A.; Corbo, M.R.; Antolak, H.; Dybka-Stępień, K.; Leszczewicz, M.; et al. Advances in Chemical and Biological Methods to Identify Microorganisms—From Past to Present. Microorganisms 2019, 7, 130. [Google Scholar] [CrossRef] [Green Version]
- Rhoads, D.; Wolcott, R.D.; Sun, Y.; Dowd, S. Comparison of Culture and Molecular Identification of Bacteria in Chronic Wounds. Int. J. Mol. Sci. 2012, 13, 2535–2550. [Google Scholar] [CrossRef]
- Wolcott, R.D. Biofilms and Chronic Infections. JAMA 2008, 299, 2682–2684. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Bou, G.; Fernández-Olmos, A.; Garcia, C.; Sáez-Nieto, J.A.; Valdezate, S. Bacterial identification methods in the microbiology laboratory. Enferm. Infecc. Microbiol. Clin 2011, 29, 601–608. [Google Scholar] [CrossRef]
- Dias, C.; Borges, A.; Oliveira, D.; Martínez-Murcia, A.; Saavedra, M.J.; Simões, M. Biofilms and antibiotic susceptibility of multidrug-resistant bacteria from wild animals. PeerJ 2018, 6, e4974. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.S.; Robertson, G. Bacterial and Fungal Biofilm Infections. Annu. Rev. Med. 2008, 59, 415–428. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.; Greenberg, E. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R. Biofilms and Device-Associated Infections. Emerg. Infect. Dis. 2001, 7, 277–281. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P.; Kathju, S.; Høiby, N.; Moser, C.; Costerton, J.W.; Moter, A.; Bjarnsholt, T. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol. Med. Microbiol. 2012, 65, 127–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Qu, F.; Ling, Y.; Mao, P.; Xia, P.; Chen, H.; Zhou, D. Biofilm-associated infections: Antibiotic resistance and novel therapeutic strategies. Futur. Microbiol. 2013, 8, 877–886. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.G.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Genet. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Najar, M.S.; Saldanha, C.L.; Banday, K.A. Approach to urinary tract infections. Indian J. Nephrol. 2009, 19, 129–139. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Catheter-Associated Urinary Tract Infections. 2015. Available online: https://www.cdc.gov/hai/ca_uti/uti.html (accessed on 28 January 2021).
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef] [Green Version]
- Pelling, H.; Nzakizwanayo, J.; Milo, S.; Denham, E.; Macfarlane, W.; Bock, L.; Sutton, J.M.; Jones, B. Bacterial biofilm formation on indwelling urethral catheters. Lett. Appl. Microbiol. 2019, 68, 277–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, M.; Al-Hasan, M.N. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin. Microbiol. Infect. 2013, 19, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, H.; Maeda, M.; Nagatomo, Y.; Takuma, T.; Niki, Y.; Naito, Y.; Sasaki, T.; Ishino, K. Epidemiology and risk factors for mortality in bloodstream infections: A single-center retrospective study in Japan. Am. J. Infect. Control. 2018, 46, e75–e79. [Google Scholar] [CrossRef]
- Kochanek, K.D.; Murphy, S.L.; Xu, J.; Arias, E. Deaths: Final data for National vital statistics reports. Atlanta Cent. Dis. Control Prev. 2019, 68, 1–77. [Google Scholar]
- Viscoli, C. Bloodstream Infections: The peak of the iceberg. Virulence 2016, 7, 248–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco-Paredes, C. Chapter 2—Bloodstream infections. In Core Concepts in Clinical Infectious Diseases (CCCID); Franco-Paredes, C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 9–15. [Google Scholar]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, F.; Kjellerup, B.V. Elimination of Bloodstream Infections Associated with Candida albicans Biofilm in Intravascular Catheters. Pathogens 2015, 4, 457–469. [Google Scholar] [CrossRef] [Green Version]
- Yousif, A.; Jamal, M.A.; Raad, I. Biofilm-Based Central Line-Associated Bloodstream Infections. In Biofilm-Based Healthcare-Associated Infections; Advances in Experimental Medicine and Biology; Donelli, G., Ed.; Springer: Cham, Switzerland, 2014; Volume 830, pp. 157–179. [Google Scholar] [CrossRef]
- Diekema, D.J.; Hsueh, P.-R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.; Jones, R.N. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [Green Version]
- Guinea, J. Global trends in the distribution of Candida species causing candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef]
- Roberts, A.E.; Kragh, K.N.; Bjarnsholt, T.; Diggle, S.P. The Limitations of In Vitro Experimentation in Understanding Biofilms and Chronic Infection. J. Mol. Biol. 2015, 427, 3646–3661. [Google Scholar] [CrossRef]
- Klevens, R.M. Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.R.; Fouts, D.E.; Archer, G.L.; Mongodin, E.F.; DeBoy, R.T.; Ravel, J.; Paulsen, I.T.; Kolonay, J.F.; Brinkac, L.; Beanan, M.; et al. Insights on Evolution of Virulence and Resistance from the Complete Genome Analysis of an Early Methicillin-Resistant Staphylococcus aureus Strain and a Biofilm-Producing Methicillin-Resistant Staphylococcus epidermidis Strain. J. Bacteriol. 2005, 187, 2426–2438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, B.J.; Tomasz, A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J. Bacteriol. 1984, 158, 513–516. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 18033. [Google Scholar] [CrossRef]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.; Eichenberger, E.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Genet. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Methicillin-Resistant Staphylococcus aureus. Available online: https://www.cdc.gov/mrsa/lab/ (accessed on 6 February 2019).
- Hetem, D.J.; Rooijakkers, S.H.M.; Ekkelenkamp, M.B. 176—Staphylococci and Micrococci. In Infectious Diseases, 4th ed.; Cohen, J., Powderly, W.G., Opal, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1509–1522. [Google Scholar]
- Alipour, F.; Ahmadi, M.; Javadi, S. Evaluation of different methods to detect methicillin resistance in Staphylococcus aureus (MRSA). J. Infect. Public Health 2014, 7, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohajeri, P.; Rezaei, M.; Farahani, A.; Gholamine, B.; Abbasi, H. Comparison of different phenotypic and genotypic methods for the detection of methicillin-resistant Staphylococcus aureus. N. Am. J. Med. Sci. 2013, 5, 637. [Google Scholar] [CrossRef]
- Koupahi, H.; Jahromy, S.H.; Rahbar, M. Evaluation of Different Phenotypic and Genotypic Methods for Detection of Methicillin Resistant Staphylococcus aureus (MRSA). Iran. J. Pathol. 2016, 11, 370–376. [Google Scholar]
- Pillai, M.M.; Latha, R.; Sarkar, G. Detection of Methicillin Resistance in Staphylococcus aureus by Polymerase Chain Reaction and Conventional Methods: A Comparative Study. J. Lab. Physicians 2012, 4, 083–088. [Google Scholar] [CrossRef]
- Júnior, F.C.S.; Néri, G.S.; Silva, A.K.; Araújo, B.P.R.C.; Guerra, M.J.P.D.; Fernandes, M.J.B.C.; Milan, E.P.; Melo, M.C.N. Evaluation of different methods for detecting methicillin resistance in Staphylococcus aureus isolates in a university hospital located in the Northeast of Brazil. Braz. J. Microbiol. 2010, 41, 316–320. [Google Scholar] [CrossRef] [Green Version]
- Elhassan, M.M.; Ozbak, H.A.; Hemeg, H.A.; Elmekki, M.A.; Ahmed, L.M. Absence of themecA Gene in Methicillin Resistant Staphylococcus aureus Isolated from Different Clinical Specimens in Shendi City, Sudan. BioMed Res. Int. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustapha, M.; Bukar-Kolo, Y.M.; Geidam, Y.A.; Gulani, I.A. Phenotypic and genotypic detection of methicillin-resistant Staphylococcus aureus in hunting dogs in Maiduguri metropolitan, Borno State, Nigeria. Veter. World 2016, 9, 501–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wang, Z.; Yan, Z.; Wu, J.; Ali, T.; Li, J.; Lv, Y.; Han, B. Bovine mastitis Staphylococcus aureus: Antibiotic susceptibility profile, resistance genes and molecular typing of methicillin-resistant and methicillin-sensitive strains in China. Infect. Genet. Evol. 2015, 31, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Kim, C.K.; Jang, J.-H.; Sung, H.; Choi, Y.; Kim, M.-N. Impact of Community-Onset Methicillin-Resistant Staphylococcus aureus Bacteremia in a Central Korea Veterans Health Service Hospital. Ann. Lab. Med. 2019, 39, 158–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashem, A.; El Fadeal, N.A.; Shehata, A. In vitro activities of vancomycin and linezolid against biofilm-producing methicillin-resistant staphylococci species isolated from catheter-related bloodstream infections from an Egyptian tertiary hospital. J. Med. Microbiol. 2017, 66, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Maor, Y.; Lago, L.; Zlotkin, A.; Nitzan, Y.; Belausov, N.; Ben-David, D.; Keller, N.; Rahav, G. Molecular features of heterogeneous vancomycin-intermediate Staphylococcus aureus strains isolated from bacteremic patients. BMC Microbiol. 2009, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guembe, M.; Alonso, B.; Lucio, J.; Granda, M.J.P.; Cruces, R.; Sánchez-Carrillo, C.; Fernández-Cruz, A.; Bouza, E. Biofilm production is not associated with poor clinical outcome in 485 patients with Staphylococcus aureus bacteraemia. Clin. Microbiol. Infect. 2018, 24, 659.e1–659.e3. [Google Scholar] [CrossRef] [Green Version]
- Klingenberg, C.; Aarag, E.; Rønnestad, A.; Sollid, J.E.; Abrahamsen, T.G.; Kjeldsen, G.; Flaegstad, T. Coagulase-Negative Staphylococcal Sepsis in Neonates. Pediatr. Infect. Dis. J. 2005, 24, 817–822. [Google Scholar] [CrossRef]
- Côrtes, M.F.; Beltrame, C.O.; Ramundo, M.S.; Ferreira, F.A.; Figueiredo, A.M.S. The influence of different factors including fnbA and mecA expression on biofilm formed by MRSA clinical isolates with different genetic backgrounds. Int. J. Med. Microbiol. 2015, 305, 140–147. [Google Scholar] [CrossRef]
- De, A.; Jorgensen, A.N.; Beatty, W.L.; Lemos, J.; Wen, Z.T. Deficiency of MecA in Streptococcus mutans Causes Major Defects in Cell Envelope Biogenesis, Cell Division, and Biofilm Formation. Front. Microbiol. 2018, 9, 2130. [Google Scholar] [CrossRef]
- Fariña, N.; Samudio, M.; Carpinelli, L.; Nentwich, M.M.; De Kaspar, H.M. Methicillin resistance and biofilm production of Staphylococcus epidermidis isolates from infectious and normal flora conjunctiva. Int. Ophthalmol. 2016, 37, 819–825. [Google Scholar] [CrossRef]
- McCarthy, H.; Rudkin, J.; Black, N.; Egallagher, L.; O’Neill, E.; O’Gara, J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2015, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Gao, H.-Y.; Li, D.; Li, Z.; Qi, S.-S.; Zheng, S.; Bai, C.-S.; Zhang, S.-H. Clinical outcome of Escherichia coli bloodstream infection in cancer patients with/without biofilm formation: A single-center retrospective study. Infect. Drug Resist. 2019, 12, 359–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaikh, S.; Fatima, J.; Shakil, S.; Rizvi, S.M.D.; Kamal, M.A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Melzer, M.; Petersen, I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. J. Infect. 2007, 55, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, G.; Fouzas, S.; Giormezis, N.; Giannakopoulos, I.; Tzifas, S.; Foka, A.; Anastassiou, D.; Spiliopoulou, I.; Mantagos, S. Clinical and microbiological profile of persistent coagulase-negative staphylococcal bacteraemia in neonates. Clin. Microbiol. Infect. 2011, 17, 1684–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agnelli, C.; Valerio, M.; Bouza, E.; Vena, A.; Guinea, J.; del Carmen Martínez-Jiménez, M.; Marcos-Zambrano, L.J.; Escribano, P.; Muñoz, P.; on behalf of the COMIC Study Group (Collaborative Group on Mycosis). Persistent Candidemia in adults: Underlying causes and clinical significance in the antifungal stewardship era. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 607–614. [Google Scholar] [CrossRef]
- Li, W.-S.; Chen, Y.-C.; Kuo, S.-F.; Chen, F.-J.; Lee, C.-H. The Impact of Biofilm Formation on the Persistence of Candidemia. Front. Microbiol. 2018, 9, 1196. [Google Scholar] [CrossRef] [Green Version]
- Monfredini, P.M.; Souza, A.C.R.; Cavalheiro, R.P.; Siqueira, R.A.; Colombo, A.L. Clinical impact of Candida spp. biofilm production in a cohort of patients with candidemia. Med. Mycol. 2017, 56, 803–808. [Google Scholar] [CrossRef]
- Muñoz, P.; Agnelli, C.; Guinea, J.; Vena, A.; Alvarez-Uria, A.; Marcos-Zambrano, L.; Escribano, P.; Valerio, M.; Bouza, E. Is biofilm production a prognostic marker in adults with candidaemia? Clin. Microbiol. Infect. 2018, 24, 1010–1015. [Google Scholar] [CrossRef] [Green Version]
- Pongrácz, J.; Benedek, K.; Juhász, E.; Iván, M.; Kristóf, K. In vitro biofilm production of Candida bloodstream isolates: Any association with clinical characteristics? J. Med. Microbiol. 2016, 65, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Koehler, P.; Stecher, M.; Cornely, O.; Vehreschild, M.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J. Morbidity and mortality of candidaemia in Europe: An epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Rajendran, R.; Sherry, L.; Nile, C.; Sherriff, A.; Johnson, E.; Hanson, M.; Williams, C.; Munro, C.; Jones, B.; Ramage, G. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection—Scotland, 2012–2013. Clin. Microbiol. Infect. 2016, 22, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsay, S.V.; Mu, Y.; Williams, S.; Epson, E.; Nadle, J.; Bamberg, W.M.; Barter, D.M.; Johnston, H.L.; Farley, M.M.; Harb, S.; et al. Burden of Candidemia in the United States, 2017. Clin. Infect. Dis. 2020, 71, 449–453. [Google Scholar] [CrossRef]
- Martínez, J.A.; Soto, S.; Fabrega, A.; Almela, M.; Mensa, J.; Soriano, A.; Marco, F.; de Anta, M.T.J.; Vila, J. Relationship of Phylogenetic Background, Biofilm Production, and Time to Detection of Growth in Blood Culture Vials with Clinical Variables and Prognosis Associated with Escherichia coli Bacteremia. J. Clin. Microbiol. 2006, 44, 1468–1474. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.-Y.; Chen, H.-L.; Huang, C.-T.; Su, L.-H.; Chiu, C.-H. Biofilm production, use of intravascular indwelling catheters and inappropriate antimicrobial therapy as predictors of fatality in Chryseobacterium meningosepticum bacteraemia. Int. J. Antimicrob. Agents 2010, 36, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.; Egger, M.; Moher, D. Addressing Reporting Biases. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley: Hoboken, NJ, USA, 2008; pp. 297–333. [Google Scholar] [CrossRef]
- Karigoudar, R.M.; Karigoudar, M.H.; Wavare, S.M.; Mangalgi, S.S. Detection of biofilm among uropathogenic Escherichia coli and its correlation with antibiotic resistance pattern. J. Lab. Physicians 2019, 11, 017–022. [Google Scholar] [CrossRef]
- Kadry, A.; Al-Kashef, N.M.; El-Ganiny, A.M. Distribution of genes encoding adhesins and biofilm formation capacity among Uropathogenic Escherichia coli isolates in relation to the antimicrobial resistance. Afr. Health Sci. 2020, 20, 238–247. [Google Scholar] [CrossRef]
- Alves, M.J.; Barreira, J.C.M.; Carvalho, I.; Trinta, L.; Perreira, L.; Ferreira, I.C.F.R.; Pintado, M.M. Propensity for biofilm formation by clinical isolates from urinary tract infections: Developing a multifactorial predictive model to improve antibiotherapy. J. Med. Microbiol. 2014, 63, 471–477. [Google Scholar] [CrossRef]
- Shrestha, R.; Khanal, S.; Poudel, P.; Khadayat, K.; Ghaju, S.; Bhandari, A.; Lekhak, S.; Pant, N.D.; Sharma, M.; Marasini, B.P. Extended spectrum β-lactamase producing uropathogenic Escherichia coli and the correlation of biofilm with antibiotics resistance in Nepal. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Raya, S.; Belbase, A.; Dhakal, L.; Prajapati, K.G.; Baidya, R.; Bimali, N.K. In-Vitro Biofilm Formation and Antimicrobial Resistance of Escherichia coli in Diabetic and Nondiabetic Patients. BioMed Res. Int. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, D.; Thapa, P.; Bhandari, D.; Parajuli, H.; Chaudhary, P.; Thapa, K.; Sharma, V.K.; Shah, P.K. Biofilm Production and Antimicrobial Resistance among Uropathogens in Pediatric Cases: A Hospital Based Study. J. Nepal Health Res. Counc. 2018, 16, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Mobley, H.; Hagan, E.C.; Donnenberg, M. Uropathogenic Escherichia coli. EcoSal Plus 2009, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoban, D.J.; Nicolle, L.E.; Hawser, S.; Bouchillon, S.; Badal, R. Antimicrobial susceptibility of global inpatient urinary tract isolates of Escherichia coli: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program: 2009–2010. Diagn. Microbiol. Infect. Dis. 2011, 70, 507–511. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, S.-J.; Choe, H.-S. Community-Acquired Urinary Tract Infection by Escherichia coli in the Era of Antibiotic Resistance. BioMed Res. Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kot, B. Antibiotic Resistance among Uropathogenic Escherichia coli. Pol. J. Microbiol. 2019, 68, 403–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudinha, T.; Johnson, J.R.; Andrew, S.D.; Kong, F.; Anderson, P.; Gilbert, G.L. Escherichia coli Sequence Type 131 as a Prominent Cause of Antibiotic Resistance among Urinary Escherichia coli Isolates from Reproductive-Age Women. J. Clin. Microbiol. 2013, 51, 3270–3276. [Google Scholar] [CrossRef] [Green Version]
- Nicolas-Chanoine, M.-H.; Bertrand, X.; Madec, J.-Y. Escherichia coli ST131, an Intriguing Clonal Group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef] [Green Version]
- Johnson, J.R.; Johnston, B.; Clabots, C.; Kuskowski, M.A.; Castanheira, M. Escherichia coliSequence Type ST131 as the Major Cause of Serious Multidrug-Resistant E. coli Infections in the United States. Clin. Infect. Dis. 2010, 51, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Mohan, B.; Taneja, N. Biofilm Formation Capability of Enterococcal Strains Causing Urinary Tract Infection vis-a-vis Colonisation and Correlation with Enterococcal Surface Protein Gene. Indian J. Med. Microbiol. 2017, 35, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Khodadadian, R.; Rahdar, H.A.; Javadi, A.; Safari, M.; Khorshidi, A. Detection of VIM-1 and IMP-1 genes in Klebsiella pneumoniae and relationship with biofilm formation. Microb. Pathog. 2018, 115, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Katouli, M.; Karimi, S. Biofilm production among methicillin resistant Staphylococcus aureus strains isolated from catheterized patients with urinary tract infection. Microb. Pathog. 2016, 98, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.B.; Baral, R.; Khanal, B. Comparative study of antimicrobial resistance and biofilm formation among Gram-positive uropathogens isolated from community-acquired urinary tract infections and catheter-associated urinary tract infections. Infect. Drug Resist. 2019, 12, 957–963. [Google Scholar] [CrossRef] [Green Version]
- Bardoloi, V.; Babu, K.V.Y. Comparative study of isolates from community-acquired and catheter-associated urinary tract infections with reference to biofilm-producing property, antibiotic sensitivity and multi-drug resistance. J. Med. Microbiol. 2017, 66, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Trautner, B.W.; Darouiche, R.O. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 2004, 32, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Soto, S.M. Importance of Biofilms in Urinary Tract Infections: New Therapeutic Approaches. Adv. Biol. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, A.L.; The PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- GraphPad. Analyze a 2 × 2 Contingency Table. Available online: https://www.graphpad.com/quickcalcs/contingency1/ (accessed on 6 June 2021).
- Peters, B.M.; Jabra-Rizk, M.A.; O’May, G.A.; Costerton, J.W.; Shirtliff, M.E. Polymicrobial Interactions: Impact on Pathogenesis and Human Disease. Clin. Microbiol. Rev. 2012, 25, 193–213. [Google Scholar] [CrossRef] [Green Version]
- Mancini, N.; Carletti, S.; Ghidoli, N.; Cichero, P.; Burioni, R.; Clementi, M. The Era of Molecular and Other Non-Culture-Based Methods in Diagnosis of Sepsis. Clin. Microbiol. Rev. 2010, 23, 235–251. [Google Scholar] [CrossRef] [Green Version]
- Tsalik, E.L.; Bonomo, R.A.; Fowler, V.G. New Molecular Diagnostic Approaches to Bacterial Infections and Antibacterial Resistance. Annu. Rev. Med. 2018, 69, 379–394. [Google Scholar] [CrossRef]
- Macia, M.; Rojo-Molinero, E.; Oliver, A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin. Microbiol. Infect. 2014, 20, 981–990. [Google Scholar] [CrossRef] [Green Version]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef] [Green Version]
- Coenye, T.; Goeres, D.; Van Bambeke, F.; Bjarnsholt, T. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin. Microbiol. Infect. 2018, 24, 570–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Vanepps, J.S.; Younger, J.G. Implantable Device-Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, J.; Azevedo, N.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hebraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2016, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.A.; Gollan, B.; Helaine, S. Persistent bacterial infections and persister cells. Nat. Rev. Genet. 2017, 15, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, E.V.T.; Lewenza, S.; Reckseidler-Zenteno, S. Current Research Approaches to Target Biofilm Infections. Postdoc J. 2015, 3, 36–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Stanton, T.B. A call for antibiotic alternatives research. Trends Microbiol. 2013, 21, 111–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2014, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Hughes, G.; Webber, M. Novel approaches to the treatment of bacterial biofilm infections. Br. J. Pharmacol. 2017, 174, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Prospects for Anti-Biofilm Pharmaceuticals. Pharmaceuticals 2015, 8, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verderosa, A.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies against Microbial Biofilm Challenges. Front. Cell. Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef]
- Weinstein, M.C.; Russell, L.B.; Gold, M.R.; Siegel, J.E. Cost-Effectiveness in Health and Medicine; Oxford University Press: Oxford, UK, 1996. [Google Scholar]











| Inclusion Criteria |
|---|
| - Observational study and original research |
| - Only Human BSI/bacteremia/fungemia/sepsis clinical isolates (BSI); Only Human UTI clinical isolates (UTI) |
| - Minimum of 15 clinical isolates (sample size) |
| - Isolates from blood cultures and/or catheter tips (BSI); Isolates from urine or catheters (UTI) |
| - Reports on biofilm in vitro production prevalence |
| - Reports on biofilm in vitro production prevalence related to clinical outcomes or to resistant vs. susceptible strains |
| - Healthcare settings (BSI); Healthcare settings (outpatients and inpatients) (UTI) |
| - In vitro biofilm production/detection only |
| - Crystal violet/safranin assay and on microtiter/tissue culture plates for biofilm production/detection * |
| - Biofilm formation in 24 h * |
| - Results in categorical data (Optical density (OD) cut-offs) |
| - OD cut-offs for negative/positive biofilm production * |
| - Studies published in English, French or Portuguese and from 1 January 2005 |
| Exclusion Criteria |
| - Contaminant isolates |
| - Results in OD mean values |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, H.; Simões, M.; Borges, A. Prevalence and Impact of Biofilms on Bloodstream and Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 825. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10070825
Pinto H, Simões M, Borges A. Prevalence and Impact of Biofilms on Bloodstream and Urinary Tract Infections: A Systematic Review and Meta-Analysis. Antibiotics. 2021; 10(7):825. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10070825
Chicago/Turabian StylePinto, Henrique, Manuel Simões, and Anabela Borges. 2021. "Prevalence and Impact of Biofilms on Bloodstream and Urinary Tract Infections: A Systematic Review and Meta-Analysis" Antibiotics 10, no. 7: 825. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics10070825