Antiplasmodial Cyclodecapeptides from Tyrothricin Share a Target with Chloroquine
Abstract
:1. Introduction
2. Results
2.1. P. falciparum Strain Susceptibility and Cytotoxicity of TrcA, PhcA and TpcC
2.2. Evaluation of Antimalarial Activity of the Cyclodecapeptides in Combination with Chloroquine
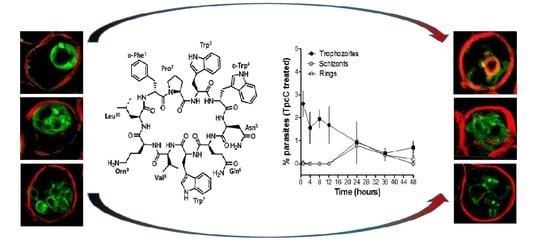
2.3. Microscopic Visualization of the Effect of Tryptocidine C on Parasite Morphology
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Parasite Culturing
4.2.2. Peptide Preparation
4.2.3. Determination of Antimalarial and Hemolytic Activities of Peptides
4.2.4. Determination of Toxicity
4.2.5. Assessment of Dose–Response Data
4.2.6. Interaction between Chloroquine and Selected Tyrocidine Analogues
4.2.7. Evaluation of TpcC Activity Using Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Global Collaborator Group, World Malaria Report 2021: An In-Depth Update on Global and Regional Malaria Data and Trends. World Health Organization Press. Available online: www.mmv.org/newsroom/publications/world-malaria-report-2012 (accessed on 28 February 2022).
- Galal, S. Coronavirus Deaths in Africa as of November 22, 2021, by Country. 2021. Available online: www.statista.com/statistics/1170530/coronavirus-deaths-in-africa (accessed on 28 February 2022).
- Payne, D. Spread of chloroquine resistance in Plasmodium Falciparum. Parasitol. Today 1987, 3, 241–246. [Google Scholar] [CrossRef]
- Jiang, H.; Joy, D.A.; Furuya, T.; Su, X. Current understanding of the molecular basis of chloroquine-resistance in Plasmodium falciparum. J. Postgr. Med. 2006, 52, 271–276. [Google Scholar]
- Campbell, C.C. Malaria control-addressing challenges to ambitious goals. N. Engl. J. Med. 2009, 361, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Henriques, G.; Martinelli, A.; Rodrigues, L.; Modrzynska, K.; Fawcett, R.; Houston, D.R.; Borges, S.T.; D’Alessandro, U.; Tinto, H.; Karema, C.; et al. Artemisinin resistance in rodent malaria-mutation in the AP2 adaptor mu-chain suggests involvement of endocytosis and membrane protein trafficking. Malar. J. 2013, 12, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jambou, R.; Legrand, E.; Niang, M.; Khim, N.; Lim, P.; Volney, B.; Ekala, M.T.; Bouchier, C.; Esterre, P.; Fandeur, T.; et al. Resistance of Plasmodium falciparum field isolates to in-vitro artemether and point mutations of the SERCA-type PfATPase6. Lancet 2005, 366, 1960–1963. [Google Scholar] [CrossRef]
- Pirahmadi, S.; Zakeri, S.; Afsharpad, M.; Djadid, N.D. Mutation analysis in pfmdr1 and pfmrp1 as potential candidate genes for artemisinin resistance in Plasmodium falciparum clinical isolates 4 years after implementation of artemisinin combination therapy in Iran. Infect. Genet. Evol. 2013, 14, 327–334. [Google Scholar] [CrossRef]
- Klein, E.Y. Antimalarial drug resistance: A review of the biology and strategies to delay emergence and spread. Int. J. Antimicrob. Agents 2013, 41, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Sherman, I.W.; Prudhomme, J.; Tait, J.F. Altered membrane phospholipid asymmetry in Plasmodium falciparum-infected erythrocytes. Parasitol. Today 1997, 13, 242–243. [Google Scholar] [CrossRef]
- Sherman, I.W.; Winograd, E. Antigens on the Plasmodium falciparum infected erythrocyte surface are not parasite derived. Parasitol. Today 1990, 6, 317–320. [Google Scholar] [CrossRef]
- Maguire, P.A.; Sherman, I.W. Phospholipid composition, cholesterol content and cholesterol exchange in Plasmodium falciparum-infected red cells. Mol. Biochem. Parasitol. 1990, 38, 105–112. [Google Scholar] [CrossRef]
- Sherman, I.W.; Greenan, J.R.T. Altered red cell membrane fluidity during schizogonic development malarial parasites Plasmodium falciparum and P. lophurae. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 641–644. [Google Scholar] [CrossRef]
- Ward, G.E.; Miller, L.H.; Dvorak, J.A. The origin of parasitophorous vacuole membrane lipids in malaria-infected erythrocytes. J. Cell Sci. 1993, 106, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.J.; Sawyer, W.H. Changes in the membrane microviscosity of mouse red blood cells infected with Plasmodium berghei detected using n-9-anthroyloxy fatty acid fluorescent probes. Parasitology 2009, 80, 331. [Google Scholar] [CrossRef]
- Allred, D.R.; Sterling, C.R.; Morse, P.D. Increased fluidity of Plasmodium berghei-infected mouse red blood cell membranes detected by electron spin resonance spectroscopy. Mol. Biochem. Parasitol. 1983, 7, 27–29. [Google Scholar] [CrossRef]
- Taraschi, T.; Parashar, A.; Hooks, M.; Rubin, H. Perturbation of red cell membrane structure during intracellular maturation of Plasmodium falciparum. Science 1986, 232, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.; Kutncr, S.; Zangwil, M.; Cabantchik, Z.I. Selectivity properties of pores induced in host erythrocyte membrane by Plasmodium falciparum. Effect of parasite maturation. Biochim. Biophys. Acta 1986, 861, 194–196. [Google Scholar] [CrossRef]
- Kutner, S.; Baruch, D.; Ginsburg, H.; Cabantchik, Z.I. Alterations in membrane permeability of malaria-infected human erythrocytes are related to the growth stage of the parasite. Biochim. Biophys. Acta 1982, 687, 113–117. [Google Scholar] [CrossRef]
- Ginsburg, H.; Kutner, S.; Krugliak, M.; Cabantchik, Z.I. Characterization of permeation pathways appearing in the host membrane of Plasmodium falciparum infected red blood cells. Mol. Biochem. Parasitol. 1985, 14, 313–322. [Google Scholar] [CrossRef]
- Kutner, S.; Ginsburg, H.; Cabantchik, Z.I. Permselectivity changes in malaria Plasmodium falciparum infected human red blood cell membranes. J. Cell. Physiol. 1983, 114, 245–251. [Google Scholar] [CrossRef]
- Ginsburg, H.; Krugliak, M.; Eidelman, O.; Cabantchik, Z.I. New permeability pathways induced in membranes of Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 1983, 8, 177–190. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Sharma, J.; Sahal, D. Anti-plasmodial action of de novo designed, cationic, lysine-branched, amphipathic, helical peptides. Malar. J. 2012, 11, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelhaus, C.; Jacobs, T.; Andrä, J.; Leippe, M. The antimicrobial peptide NK-2, the core region of mammalian NK-lysin, kills intraerythrocytic Plasmodium falciparum. Antimicrob. Agents Chemother. 2008, 52, 1713–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, L.L.; Howard, R.J.; Aikawat, M.; Taraschi, T.F. Modification of host cell membrane lipid composition by the intra-erythrocytic human malaria parasite. Biochem. J. 1991, 274, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiehart, U.I.M.; Rautenbach, M.; Hoppe, H.C. Selective lysis of erythrocytes infected with the trophozoite stage of Plasmodium falciparum by polyene macrolide antibiotics. Biochem. Pharmacol. 2006, 71, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Rotem, S.; Mor, A. Antimicrobial peptide mimics for improved therapeutic properties. Biochim. Biophys. Acta 2009, 1788, 1582–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taliaferro, L.G.; Coulston, F.; Silvermanm, M. The antimalarial activity of tyrothricin against Plasmodium gallinaceum. J. Infect. Dis. 1944, 75, 179–211. [Google Scholar] [CrossRef]
- Otten-Kuipers, M.A.; Roelofsen, B.; Op den Kamp, J.A.F. Stage-dependent effects of analogs of gramicidin A on the growth of Plasmodium falciparum in vitro. Parasitol. Res. 1995, 81, 26–31. [Google Scholar] [CrossRef]
- Otten-Kuipers, M.A. Coppens-Burkunk, G.W.M.; Kronenburg, N.A.; Braga Fernandes Vis, M.A. Roelofsen, B.; Op den Kamp, J.A.F. Tryptophan-N-formylated gramicidin causes growth inhibition of Plasmodium falciparum by induction of potassium efflux from infected erythrocytes. Parasitol. Res. 1997, 83, 185–192. [Google Scholar] [CrossRef]
- Zorzi, A.; Deyle, K.; Heinis, C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017, 38, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Portmann, C.; Blom, J.F.; Kaiser, M.; Brun, R.; Jüttner, F.; Gademann, K. Isolation of aerucyclamides C and D and structure revision of microcyclamide 7806A: Heterocyclic ribosomal peptides from Microcystis aeruginosa PCC 7806 and their antiparasite evaluation. J. Nat. Prod. 2008, 71, 1891–1896. [Google Scholar] [CrossRef]
- Jang, J.-P.; Nogawa, T.; Futamura, Y.; Shimizu, T.; Hashizume, D.; Takahashi, S.; Jang, J.-H.; Ahn, J.S.; Osada, H. Octaminomycins A and B, cyclic octadepsipeptides active against Plasmodium falciparum. J. Nat. Prod. 2017, 80, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Buerstner, N.; Roggo, S.; Ostermann, N.; Blank, J.; Delmas, C.; Freuler, F.; Gerhartz, B.; Hinninger, A.; Hoepfner, D.; Liechty, B.; et al. Gift from nature: Cyclomarin A kills mycobacteria and malaria parasites by distinct modes of action. ChemBioChem 2015, 16, 2433–2436. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.J.; Thibault, P.; Boyd, R.K. Characterisation of the tyrocidine and gramicidin fractions of the tyrothricin complex from Bacillus brevis using liquid chromatography and mass spectrometry. Int. J. Mass Spectrom. Ion Processes 1992, 122, 153–179. [Google Scholar] [CrossRef]
- Rautenbach, M.; Vlok, N.M.; Stander, M.; Hoppe, H.C. Inhibition of malaria parasite blood stages by tyrocidines, membrane-active cyclic peptide antibiotics from Bacillus Brevis. Biochim. Biophys. Acta 2007, 1768, 1488–1497. [Google Scholar] [CrossRef] [Green Version]
- Berditsch, M.; Afonin, S.; Ulrich, A.S. The ability of Aneurinibacillus migulanus (Bacillus brevis) to produce the antibiotic gramicidin S is correlated with phenotype variation. Appl. Environ. Microbiol. 2007, 7320, 6620–6628. [Google Scholar] [CrossRef] [Green Version]
- Jokonya, S.; Langlais, M.; Leshabane, M.; Reader, P.W.; Vosloo, J.A.; Pfukwa, R.; Coertzen, D.; Birkholtz, L.; Rautenbach, M.; Klumperman, B. 2020. Poly-N-vinylpyrrolidone antimalaria conjugates of membrane-disruptive peptides. Biomacromolecules 2020, 21, 5053–5066. [Google Scholar] [CrossRef]
- Zhang, J.; Krugliak, M.; Ginsburg, H. The fate of ferriprotorphyrin IX in malaria infected erythrocytes in conjunction with the mode of action of antimalarial drugs. Mol. Biochem. Parasitol. 1999, 99, 129–141. [Google Scholar] [CrossRef]
- Orjih, A.U.; Chevli, R.; Fitch, C.D. Toxic heme in sickle cells: An explanation for death of malaria parasites. Am. J. Trop. Med. Hyg. 1985, 342, 223–227. [Google Scholar] [CrossRef]
- Villiers, K.A. De, Egan, T.J.; de Villiers, K.A.; Egan, T.J. Recent advances in the discovery of haem-targeting drugs for malaria and schistosomiasis. Molecules 2009, 14, 2868–2887. [Google Scholar] [CrossRef]
- Macomber, P.B.; Sprinz, H.; Tousimis, A.J. Morphological effects of chloroquine on Plasmodium berghei in mice. Nature 1967, 214, 937–939. [Google Scholar] [CrossRef]
- Warhurst, D.C.; Hockley, D.J. Mode of action of chloroquine on Plasmodium berghei and P. cynomolgi. Nature 1967, 214, 935–936. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, J.M.; Coppens, I.; Tripathi, A.K.; Scholl, P.F.; Shuman, J.; Bajad, S.; Shulaev, V.; Sullivan, D.J. The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochem. J. 2007, 402, 197–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, T.J. Haemozoin formation. Mol. Biochem. Parasitol. 2008, 157, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Egan, T.J. Neutral lipids associated with haemozoin mediate efficient and rapid β-haematin formation at physiological pH, temperature and ionic composition. Malar. J. 2012, 11, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapishnikov, S.; Weiner, A.; Shimoni, E.; Guttmann, P.; Schneider, G.; Dahan-Pasternak, N.; Dzikowski, R.; Leiserowitz, L.; Elbaum, M. Oriented nucleation of hemozoin at the digestive vacuole membrane in Plasmodium Falciparum. Proc. Natl. Acad. Sci. USA 2012, 109, 11188–11193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huy, N.T.; Shima, Y.; Maeda, A.; Men, T.T.; Hirayama, K.; Hirase, A.; Miyazawa, A.; Kamei, K. Phospholipid membrane-mediated hemozoin formation: The effects of physical properties and evidence of membrane surrounding hemozoin. PLoS ONE 2013, 8, e70025. [Google Scholar]
- Stiebler, R.; Soares, J.B.R.C.; Timm, B.L.; Silva, J.R.; Mury, F.B.; Dansa-Petretski, M.; Oliveira, M.F. On the mechanisms involved in biological heme crystallization. J. Bioenerg. Biomembr. 2011, 43, 93–99. [Google Scholar] [CrossRef]
- Hoang, A.N.; Sandlin, R.D.; Omar, A.; Egan, T.J.; Wright, D.W. The neutral lipid composition present in the digestive vacuole of Plasmodium falciparum concentrates heme and mediates β-hematin formation with an unusually low activation energy. Biochemistry 2010, 49, 10107–10116. [Google Scholar] [CrossRef] [Green Version]
- Holz, G.G. Lipids and the malarial parasite. Bull. World Health Org. 1977, 55, 237–248. [Google Scholar]
- Ancelin, M.L.; Vial, H.J. Saturable and non-saturable components of choline transport in Plasmodium-infected mammalian erythrocytes: Possible role of experimental conditions. Biochem. J. 1992, 283, 619–621. [Google Scholar] [CrossRef] [Green Version]
- Vielemeyer, O.; McIntosh, M.T.; Joiner, K.A.; Coppens, I. Neutral lipid synthesis and storage in the intraerythrocytic stages of Plasmodium Falciparum. Mol. Biochem. Parasitol. 2004, 135, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Nawabi, P.; Lykidis, A.; Ji, D.; Haldar, K. Neutral lipid analysis reveals elevation of acylglycerols and lack of cholesterol esters in Plasmodium falciparum-infected erythrocytes. Eukaryot. Cell 2003, 2, 1128–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kachel, K.; Asuncion-Punzalan, E.; London, E. Anchoring of tryptophan and tyrosine analogues at the hydrocarbon polar boundary in model membrane vesicles: Parallax analysis of fluorescence quenching induced by nitroxide-labeled phospholipids. Biochemistry 1995, 34, 15475–15479. [Google Scholar] [CrossRef]
- Kelkar, D.A.; Chattopadhyay, A. Membrane interfacial localization of aromatic amino acids and membrane protein function. J. Biosci. 2006, 31, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Norman, K.E.; Nymeyer, H. Indole localization in lipid membranes revealed by molecular simulation. Biophys. J. 2006, 91, 2046–2054. [Google Scholar] [CrossRef] [Green Version]
- Wymore, T.; Wong, T.C. Molecular dynamics study of substance P peptides in a biphasic membrane mimic. Biophys. J. 1999, 76, 1199–1212. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.A.; Citron, D.M.; Wang, C.C.; Citron, M. Development of tyrocidine A analogues with improved antibacterial activity. Bioorg. Med. Chem. 2007, 15, 6667–6677. [Google Scholar] [CrossRef] [Green Version]
- Dathe, M.; Wieprecht, T. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta Biomem. 1999, 1462, 71–87. [Google Scholar] [CrossRef] [Green Version]
- Giangaspero, A.; Sandri, L.; Tossi, A. Amphipathic α helical antimicrobial peptides: A systematic study of the effects of structural and physical properties on biological activity. Eur. J. Biochem. 2001, 268, 5589–5600. [Google Scholar] [CrossRef]
- Toulme, J.-J.; Charlier, M.; Helene, C. Specific recognition of single-stranded regions in ultraviolet-irradiated and heat-denatured DNA by tryptophan-containing peptides. Proc. Natl. Acad. Sci. USA 1974, 71, 3185–3188. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, M.; Rautenbach, M.; Vosloo, J.A.; Siersma, T.; Aisenbrey, C.H.M.; Zaitseva, E.; Laubscher, W.E.; van Rensburg, W.; Behrends, J.C.; Bechinger, B.; et al. The multifaceted antibacterial mechanisms of the pioneering peptide antibiotics tyrocidine and gramicidin S. mBio 2018, 9, e00802-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.E.; Kirk, K. The malaria parasite’s chloroquine resistance transporter is a member of the drug/metabolite transporter superfamily. Mol. Biol. Evol. 2004, 21, 1938–1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.P.; Dave, A.; Stein, W.D.; Lanzer, M. Transporters as mediators of drug resistance in Plasmodium Falciparum. Int. J. Parasitol. 2010, 40, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.A.; Lane, K.D.; Deng, B.; Mu, J.; Patel, J.J.; Wellems, T.E.; Su, X.; Ferdig, M.T. Mutations in transmembrane domains 1, 4 and 9 of the Plasmodium falciparum chloroquine resistance transporter alter susceptibility to chloroquine, quinine and quinidine. Mol. Microbiol. 2007, 63, 270–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sá, J.M.; Twu, O.; Hayton, K.; Reyes, S.; Fay, M.P.; Ringwald, P.; Wellems, T.E. Geographic patterns of Plasmodium falciparum drug resistance distinguished by differential responses to amodiaquine and chloroquine. Proc. Natl. Acad. Sci. USA 2009, 106, 18883–18889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.J.; Fidock, D.A.; Mungthin, M.; Lakshmanan, V.; Sidhu, A.B.S.; Bray, P.G.; Ward, S.A. Evidence for a central role for PfCRT in conferring Plasmodium falciparum resistance to diverse antimalarial agents. Mol. Cell 2004, 15, 867–877. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.E.; Marchetti, R.V.; Cowan, A.I.; Howitt, S.M.; Bröer, S.; Kirk, K. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science 2009, 325, 1680–1682. [Google Scholar] [CrossRef] [Green Version]
- Shafik, S.H.; Cobbold, S.A.; Barkat, K.; Richards, S.N.; Lancaster, N.S.; Llinás, M.; Hogg, S.J.; Summers, R.L.; McConville, M.J.; Martin, R.E. The natural function of the malaria parasite’s chloroquine resistance transporter. Nat. Commun. 2020, 11, 3922. [Google Scholar] [CrossRef]
- Fidock, D.A.; Nomura, T.; Talley, A.K.; Cooper, R.A.; Dzekunov, S.M.; Ferdig, M.T.; Ursos, L.M.; Sidhu, A.B.; Naudé, B.; Deitsch, K.W.; et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 2000, 6, 861–871. [Google Scholar] [CrossRef]
- Cowman, A.F.; Karcz, S.; Galatis, D.; Culvenor, J.G. A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J. Cell Biol. 1991, 113, 1033–1042. [Google Scholar] [CrossRef]
- Wellems, T.E.; Oduola, A.M.J.; Fenton, B.; Desjardins, R.; Panton, L.J.; DoRosario, V.E. Chromosome size variation occurs in cloned Plasmodium falciparum on in vitro cultivation. Rev. Bras. Genet. 1988, 11, 813–825. [Google Scholar]
- Yuan, J.; Johnson, R.L.; Huang, R.; Wichterman, J.; Jiang, H.; Hayton, K.; Fidock, D.A.; Wellems, T.E.; Inglese, J.; Austin, C.P.; et al. Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum. Nat. Chem. Biol. 2009, 5, 765–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.P.; Rotmann, A.; Stein, W.D.; Lanzer, M. Polymorphisms within PfMDR1 alter the substrate specificity for anti-malarial drugs in Plasmodium Falciparum. Mol. Microbiol. 2008, 70, 786–798. [Google Scholar] [PubMed]
- Simon, N.; Voigtländer, C.; Kappes, B.; Rohrbach, P.; Friedrich, O. PfMDR1 transport rates assessed in Intact Isolated Plasmodium falciparum digestive vacuoles reflect functional drug resistance relationship with pfmdr1 mutations. Pharmaceuticals 2022, 15, 202. [Google Scholar] [CrossRef]
- Kolakovich, K.A.; Gluzman, I.Y.; Duffin, K.L.; Goldberg, D.E. Generation of hemoglobin peptides in the acidic digestive vacuole of Plasmodium falciparum implicates peptide transport in amino acid production. Mol. Biochem. Parasitol. 1997, 87, 123–135. [Google Scholar] [CrossRef]
- Sharma, R.C.; Inoue, S.; Roitelman, J.; Schimke, R.T.; Simoni, R.D. Peptide transport by the multidrug resistance pump. J. Biol. Chem. 1992, 267, 5731–5734. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Borgnia, M.J. Probing the interaction of the multidrug resistance phenotype with the polypeptide ionophore gramicidin D via functional channel formation. Eur. J. Biochem. 1994, 222, 813–824. [Google Scholar] [CrossRef]
- Lincke, C.R.; Van der Bliek, A.M.; Schuurhuis, G.J.; Van der Velde-Koerts, T.; Smit, J.J.; Borst, P. Multidrug resistance phenotype of human BRO melanoma cells transfected with a wild-type human mdr1 complementary DNA. Cancer Res. 1990, 50, 1779–1785. [Google Scholar]
- Bray, P.G.; Mungthin, M.; Ridley, R.G.; Ward, S.A. Access to hematin: The basis of chloroquine resistance. Mol. Pharmacol. 1998, 54, 170–179. [Google Scholar] [CrossRef]
- Le Bras, J.; Durand, R. The mechanisms of resistance to antimalarial drugs in Plasmodium falciparum. Fundam. Clin. Pharmacol. 2003, 17, 147–153. [Google Scholar] [CrossRef]
- Wünsch, S.; Sanchez, C.P.; Gekle, M.; Grosse-Wortmann, L.; Wiesner, J.; Lanzer, M. Differential stimulation of the Na+/H+ exchanger determines chloroquine uptake in Plasmodium falciparum. J. Cell Biol. 1998, 140, 335–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliba, K.J.; Folb, P.I.; Smith, P.J. Role for the Plasmodium falciparum digestive vacuole in chloroquine resistance. Biochem. Pharmacol. 1998, 56, 313–320. [Google Scholar] [CrossRef]
- Cranmer, S.L.; Magowan, C.; Liang, J.; Coppel, R.L.; Cooke, B.M. An alternative to serum for cultivation of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 363–365. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Makowa, H.B. The Relationship between the Insecticide Dichloro-Diphenyl-Trichloroethane and Chloroquine in Plasmodium falciparum Resistance. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, March 2012. Available online: http://scholar.sun.ac.za/handle/10019.1/20310?show=full (accessed on 28 April 2022).
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Diggs, C.; Joseph, K.; Flemmings, B.; Snodgrass, R.; Hines, F. Protein synthesis in vitro by cryopreserved Plasmodium Falciparum. Am. J. Trop. Med. Hyg. 1975, 24, 760–763. [Google Scholar] [CrossRef]
- Reilly, J.T.; Bain, B.J.; Amos, R.; Cavill, I.; Chapman, C.; England, J.M.; Hyde, K.; Matutes, E.; Wood, J.K.K.; Chairman, R. The laboratory diagnosis of malaria. Clin. Lab. Haematol. 1997, 2, 165–170. [Google Scholar] [CrossRef]
- Schuster, F.L. Cultivation of Plasmodium spp. Clin. Microbiol. Rev. 2002, 15, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Leussa, A.N.-N.; Rautenbach, M. SAR and PCA analysis of the tyrocidines towards leucocin A sensitive and resistant Listeria monocytogenes. Chem. Biol. Drug Des. 2014, 84, 543–557. [Google Scholar] [CrossRef]
- Nkhoma, S.; Molyneux, M.; Ward, S. In vitro antimalarial susceptibility profile and pfcrt/pfmdr-1 genotypes of Plasmodium falciparum field isolates from Malawi. Am. J. Trop. Med. Hyg. 2007, 76, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Gomez, M.S.S.; Piper, R.C.C.; Hunsaker, L.A.A.; Royer, R.E.E.; Deck, L.M.M.; Makler, M.T.T.; Vander Jagt, D.L.; Jagt, D.L. Vander. Substrate and cofactor specificity and selective inhibition of lactate dehydrogenase from the malarial parasite P. falciparum. Mol. Biochem. Parasitol. 1997, 90, 235–246. [Google Scholar] [CrossRef]
- Schloms, L.; Storbeck, K.; Swart, P.; Gelderblom, W.C.A.; Swart, A.C. The influence of Aspalathus linearis Rooibos and dihydrochalcones on adrenal steroidogenesis: Quantification of steroid intermediates and end products in H295R cells. J. Steroid Biochem. Mol. Biol. 2012, 128, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Rautenbach, M.; Gerstner, G.D.; Vlok, N.M.; Kulenkampff, J.; Westerhoff, H.V. Analyses of dose-response curves to compare the antimicrobial activity of model cationic alpha-helical peptides highlights the necessity for a minimum of two activity parameters. Anal. Biochem. 2006, 350, 81–90. [Google Scholar] [CrossRef]
- Chawira, A.N.; Warhurst, D.C. The effect of artemisinin combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. J. Trop. Med. Hyg. 1987, 90, 1–8. [Google Scholar] [PubMed]
- Fivelman, Q.L.; Adagu, I.S.; David, C.; Warhurst, D.C. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium Falciparum. Antimicrob. Agents Chemother. 2004, 48, 4097–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A. Antimalarial drug synergism and antagonism: Mechanistic and clinical significance. FEMS Microbiol. Lett. 2005, 253, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Odds, F.C. Synergy, antagonism, what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- Gupta, S.; Thapar, M.M.; Wernsdorfer, W.H.; Björkman, A. In vitro interactions of artemisinin with atovaquone, quinine, mefloquine against Plasmodium falciparum. Antimicrob. Agents Chemother. 2002, 46, 1510–1515. [Google Scholar] [CrossRef] [Green Version]




| Compounds Tested | Toxicity (LC50, μM (n)); HC50, μM ± SEM (n) | Parasite Strains (IC50, nM ± SEM (n)) | Resistance Index [23] | Selectivity Index | |||
|---|---|---|---|---|---|---|---|
| COS Cells | Erythrocytes | CQS P. falciparum 3D7 | CQI P. falciparum D10 | CQR P. falciparum Dd2 | IC50Dd2/IC50 # | IC50/LC50 * | |
| TpcC | 10 (2) | 9.1 ± 1.5 (4) | 126 ± 28 (6) a,b,1,2,3 | 42 ± 17 (11) a,c | 398 ± 94 (3) b,c,5 | 3; 9 | 79; 238; 25 |
| TrcA | 6 (2) | 6. 1± 0.6 (5) | 41 ± 12 (6) d,1 | 119 ± 33 (10) e,4 | 1802 ± 319 (3) d,e,5,6,7 | 44; 15 | 146; 51; 3 |
| PhcA | 8 (2) | 7.1 ± 1.6 (8) | 23 ± 7 (3) f,2 | 52 ± 17 (11) g | 511 ± 96 (3) f,g,6 | 22; 10 | 384; 154; 16 |
| GS | 9 (2) | 6.2 ± 0.4 (9) | 1452 ± 151 (6) | 1398 ± 86 (16) | 1861 ± 160 (6) | 1.3; 1.3 | 6; 6; 5 |
| CQ | na | na | 17 ± 5 (4) h,i,3 | 40 ± 3 (12) h,j,l,4 | 277 ± 18 (6) i,j,7 | 16; 7 | na |
| Peptide | Combination Ratio CQ:Peptide at IC50 | CQ FIC | Peptide FIC | CQ:Peptide FIC Index * | Isobologram |
|---|---|---|---|---|---|
| Tryptocidine C | 1:2 | 0.36 ± 0.07 | 0.90 ± 0.40 | 1.26 ± 0.36 |  |
| 1:5 | 0.23 ± 0.08 | 1.24 ± 0.26 | 1.47 ± 0.28 | ||
| 1:10 | 0.08 ± 0.03 | 0.93 ± 0.16 | 1.02 ± 0.17 | ||
| Tyrocidine A | 1:1 | 1.77 ± 0.29 | 1.99 ± 0.36 | 3.76 ± 0.65 |  |
| 1:2 | 0.66 ± 0.02 | 0.95 ± 0.18 | 1.60 ± 0.20 | ||
| 1:10 | 0.13 ± 0.10 | 1.21 ± 0.29 | 1.35 ± 0.37 | ||
| Phenycidine A | 1:2 | 1.09 ± 0.03 | 2.44 ± 0.20 | 3.53 ± 0.22 |  |
| 1:5 | 0.33 ± 0.15 | 2.82 ± 0.48 | 3.15 ± 0.33 | ||
| 1:10 | 0.05 ± 0.02 | 0.49 ± 0.22 | 0.54 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leussa, A.N.-N.; Rautenbach, M. Antiplasmodial Cyclodecapeptides from Tyrothricin Share a Target with Chloroquine. Antibiotics 2022, 11, 801. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11060801
Leussa AN-N, Rautenbach M. Antiplasmodial Cyclodecapeptides from Tyrothricin Share a Target with Chloroquine. Antibiotics. 2022; 11(6):801. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11060801
Chicago/Turabian StyleLeussa, Adrienne N.-N., and Marina Rautenbach. 2022. "Antiplasmodial Cyclodecapeptides from Tyrothricin Share a Target with Chloroquine" Antibiotics 11, no. 6: 801. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics11060801