Utilizing Monte Carlo Simulations to Optimize Institutional Empiric Antipseudomonal Therapy
Abstract
:1. Introduction
2. Methods
2.1. Data Collection
2.2. Model Construction
3. Results
| Breakpoint a (mcg/mL) | MIC Range (mcg/mL) | MIC50 (mcg/mL) | MIC90 (mcg/mL) | % Susceptible | |
|---|---|---|---|---|---|
| Aztreonam | 8 | ≤2–≥32 | 8 | 32 | 68 |
| Cefepime | 8 | ≤1–≥32 | 4 | 16 | 81 |
| Meropenem | 2 | ≤1–≥16 | 1 | 8 | 74 |
| Piperacillin | 16 | ≤2–≥128 | 8 | 128 | 75 |
| Antimicrobial Agent | Clearance (L/h) | Volume of Distribution (L) | Half Life (h) | Protein Binding (%) |
|---|---|---|---|---|
| Aztreonam [16,17] | 5.45 ± 1.24 | 13.7 ± 4.94 | 1.69 ± 0.43 | 56 |
| Cefepime [18,19] | 8.58 ± 1.5 | 18.4 ± 3.8 | 2.32 ± 0.39 | 20 |
| Meropenem [15,20] | 11.28 ± 1.86 | 12.5 ± 1.5 | 0.98 | 2 |
| Piperacillin [21,22] | 11.07 ± 2.59 | 11.2 ± 2.1 | 0.7 ± 0.11 | 30 |
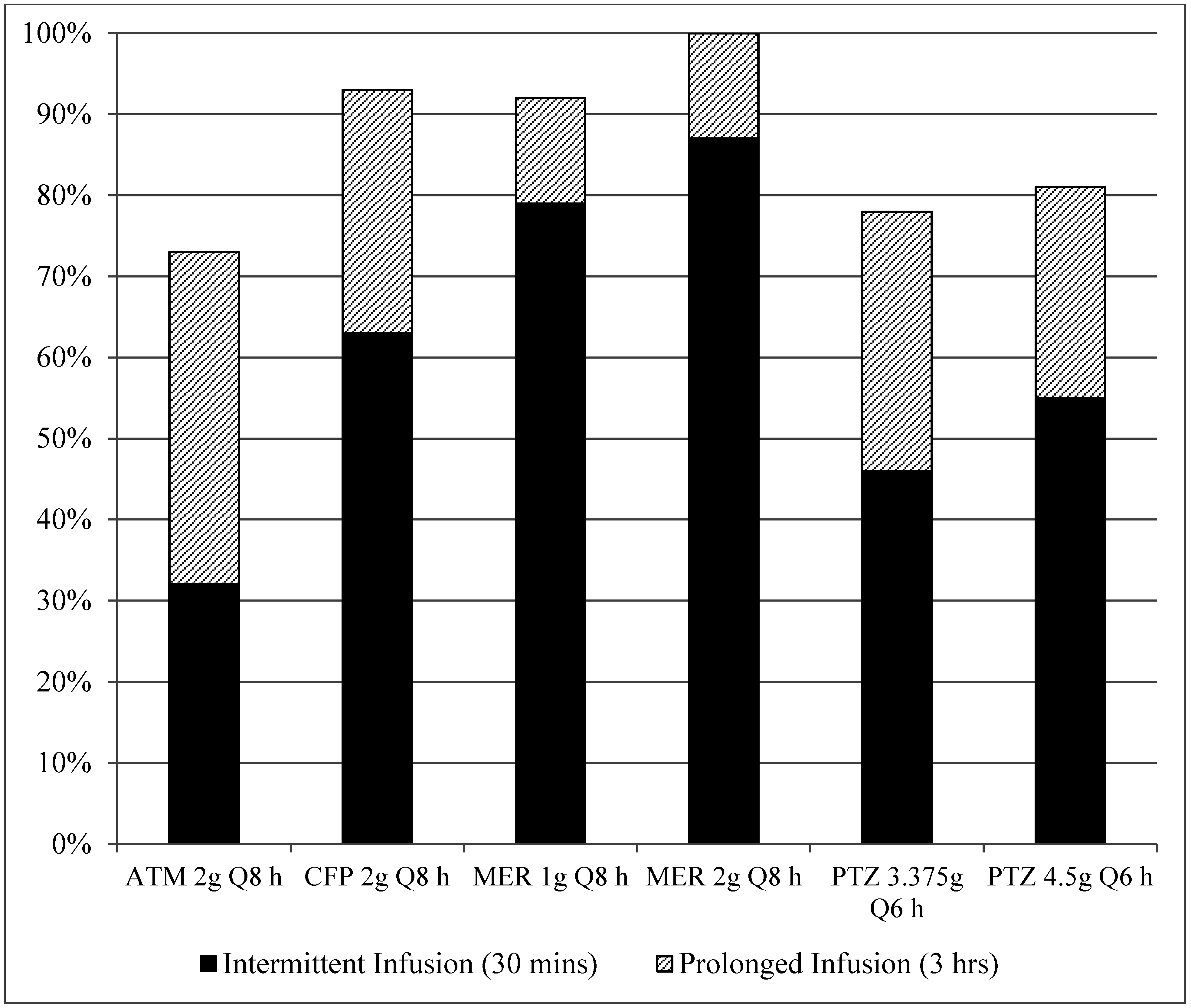
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control. Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Garnacho-Montero, J.; Sa-Borges, M.; Sole-Violan, J.; Barcenilla, F.; Escoresca-Ortega, A.; Ochoa, M.; Cayuela, A.; Rello, J. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: An observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit. Care Med. 2007, 35, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Vazquez-Guillamet, C.; Kollef, M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2014, 18, 596. [Google Scholar] [CrossRef] [PubMed]
- Lautenbach, E.; Weiner, M.G.; Nachamkin, I.; Bilker, W.B.; Sheridan, A.; Fishman, N.O. Imipenem resistance among Pseudomonas aeruginosa isolates: Risk factors for infection and impact of resistance on clinical and economic outcomes. Infect. Control Hosp. Epidemiol. 2006, 27, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Obritsch, M.D.; Fish, D.N.; MacLaren, R.; Jung, R. National surveillance of antimicrobial resistance in Pseudomonas aeruginosa isolates obtained from intensive care unit patients from 1993 to 2002. Antimicrob. Agents Chemother. 2004, 48, 4606–4610. [Google Scholar] [CrossRef] [PubMed]
- Hindler, J.F.; Stelling, J. Analysis and presentation of cumulative antibiograms: A new consensus guideline from the clinical and laboratory standards institute. Clin. Infect. Dis. 2007, 44, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Pharmacokinetic/pharmacodynamic parameters: Rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bonate, P.L. A brief introduction to Monte Carlo simulation. Clin. Pharmacokinet. 2001, 40, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Kirkpatrick, C.M.J.; Lipman, J. Monte Carlo simulations: Maximizing antibiotic pharmacokinetic data to optimize clinical practice for critically ill patients. J. Antimicrob. Chemother. 2011, 66, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Frei, C.R.; Wiederhold, N.P.; Burgess, D.S. Antimicrobial breakpoints for Gram-negative aerobic bacteria based on pharmacokinetic-pharmacodynamic models with Monte Carlo simulation. J. Antimicrob. Chemother. 2008, 61, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Shea, K.M.; Cheatham, S.C.; Wack, M.F.; Smith, D.W.; Sowinski, K.M.; Kays, M.B. Steady-state pharmacokinetics and pharmacodynamics of piperacillin/tazobactam administered by prolonged infusion in hospitalised patients. Int. J. Antimicrob. Agents 2009, 34, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- Nilsson-Ehle, I.; Hutchison, M.; Haworth, S.J.; Norrby, S.R. Pharmacokinetics of meropenem compared to imipenem-cilastatin in young, healthy males. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.R.; Wilkinson, P.; Mendelson, M.H.; Bournazos, C.; Tejero, C.; Hirschman, S.Z. Pharmacokinetics of aztreonam in healthy elderly and young adult volunteers. J. Clin. Pharmacol. 1993, 33, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb Company. Azactam (Aztreonam) Package Insert; revised; Bristol-Myers Squibb Company: Princeton, NJ, USA, 2013. [Google Scholar]
- Barbhaiya, R.H.; Forgue, S.T.; Gleason, C.R.; Knupp, C.A.; Pittman, K.A.; Weidler, D.J.; Movahhed, H.; Tenney, J.; Martin, R.R. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob. Agents Chemother. 1992, 36, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Hospira, Inc. Maxipime (Cefepime) Package Insert; revised; Hospira, Inc.: Lake Forest, IL, USA, 2014. [Google Scholar]
- AstraZeneca Pharmaceuticals LP. Merrem (Meropenem) Package Insert; revised; AstraZeneca Pharmaceuticals LP.: Wilmington, DE, USA, 2013. [Google Scholar]
- Kim, M.K.; Capitano, B.; Mattoes, H.M.; Xuan, D.; Quintiliani, R.; Nightingale, C.H.; Nicolau, D.P. Pharmacokinetic and pharmacodynamic evaluation of two dosing regimens for piperacillin-tazobactam. Pharmacotherapy 2002, 22, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Apotex Corp. Zosyn (Piperacillin and Tazobactam) Package Insert; revised; Apotex Corp.: Weston, FL, USA, 2009. [Google Scholar]
- Goff, D.A.; Nicolau, D.P. When pharmacodynamics trump costs: An antimicrobial stewardship program’s approach to selecting optimal antimicrobial agents. Clin. Ther. 2013, 35, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Nicasio, A.M.; Eagye, K.J.; Nicolau, D.P.; Shore, E.; Palter, M.; Pepe, J.; Kuti, J.L. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J. Crit. Care 2010, 25, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. M39-A4: Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014; Volume 34. [Google Scholar]
- Lodise, T.P., Jr.; Lomaestro, B.; Rodvold, K.A.; Danziger, L.H.; Drusano, G.L. Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlo simulation. Antimicrob. Agents Chemother. 2004, 48, 4718–4724. [Google Scholar] [CrossRef] [PubMed]
- Fish, D.N.; Kiser, T.H. Correlation of pharmacokinetic/pharmacodynamic-derived predictions of antibiotic efficacy with clinical outcomes in severely ill patients with Pseudomonas aeruginosa pneumonia. Pharmacotherapy 2013, 33, 1022–1034. [Google Scholar] [CrossRef] [PubMed]
- Dodds Ashley, E.S.; Kaye, K.S.; DePestel, D.D.; Hermsen, E.D. Antimicrobial stewardship: Philosophy versus practice. Clin. Infect. Dis. 2014, 59, S112–S121. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.; Hershberger, E.; Miller, B.; Popejoy, M.; Friedland, I.; Steenbergen, J.; Yoon, M.; Collins, S.; Yuan, G.; Barie, P.S.; et al. Ceftolozane/tazobactam plus metronidazole for complicated intra-abdominal infections in an era of multidrug resistance: Results from a randomized, double-blind, phase 3 trial (aspect-ciai). Clin. Infect. Dis. 2015, 60, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.; Umeh, O.; Steenbergen, J.; Yuan, G.; Darouiche, R.O. Ceftolozane-tazobactam compared with levofloxacin in the treatment of complicated urinary-tract infections, including pyelonephritis: A randomised, double-blind, phase 3 trial (aspect-cuti). Lancet 2015, 385, 1949–1956. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Gonzalez Patzan, L.D.; Stricklin, D.; Duttaroy, D.D.; Kreidly, Z.; Lipka, J.; Sable, C. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: Results of a prospective, investigator-blinded, randomized study. Curr. Med. Res. Opin. 2012, 28, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Lucasti, C.; Popescu, I.; Ramesh, M.K.; Lipka, J.; Sable, C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole vs meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: Results of a randomized, double-blind, phase II trial. J. Antimicrob. Chemother. 2013, 68, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Castanheira, M.; Mendes, R.E.; Flamm, R.K.; Farrell, D.J.; Jones, R.N. Ceftazidime-avibactam activity against multidrug-resistant Pseudomonas aeruginosa isolated in U.S. medical centers in 2012 and 2013. Antimicrob. Agents Chemother. 2015, 59, 3656–3659. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C.A.; Nicolau, D.P. Susceptibility profile of ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from US hospitals. Clin. Ther. 2015, 37, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tennant, S.J.; Burgess, D.R.; Rybak, J.M.; Martin, C.A.; Burgess, D.S. Utilizing Monte Carlo Simulations to Optimize Institutional Empiric Antipseudomonal Therapy. Antibiotics 2015, 4, 643-652. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics4040643
Tennant SJ, Burgess DR, Rybak JM, Martin CA, Burgess DS. Utilizing Monte Carlo Simulations to Optimize Institutional Empiric Antipseudomonal Therapy. Antibiotics. 2015; 4(4):643-652. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics4040643
Chicago/Turabian StyleTennant, Sarah J., Donna R. Burgess, Jeffrey M. Rybak, Craig A. Martin, and David S. Burgess. 2015. "Utilizing Monte Carlo Simulations to Optimize Institutional Empiric Antipseudomonal Therapy" Antibiotics 4, no. 4: 643-652. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics4040643





