Evaluation of a Computerized Decision Support Intervention to Decrease Use of Anti-Pseudomonal Carbapenems in Penicillin Allergic Patients
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
| Demographics and Indications | Pre-Implementation (n = 68) | Post-Implementation (n = 59) | p |
|---|---|---|---|
| Patient Demographics | |||
| Age (years), Mean (SD) | 71.4 (14.3) | 69.5 (12.4) | 0.43 |
| Male sex, n (%) | 66 (97.1%) | 58 (98.3%) | 0.64 |
| Non-ICU admission, n (%) | 41 (60.3%) | 42 (71.2%) | 0.19 |
| Duration of APC therapy, Mean (SD) | 4.4 (4.07) | 4.0 (2.60) | 0.52 |
| APC Initiations by Indication, n (%) | |||
| History of any β-lactam allergy | 41 (60.3%) | 32 (54.2%) | 0.59 |
| β-lactam allergy (high-risk) | 3 (7.3%) | 6 (18.7%) | 0.17 |
| β-lactam allergy (low-risk) | 38 (92.7%) | 26 (81.3%) | 0.17 |
| β-lactam use within past 90 days | 15 (22.1%) | 14 (23.7%) | 0.99 |
| Severe infection, pancreatitis, and/or sepsis | 19 (27.9%) | 24 (40.7%) | 0.19 |
| Suspected/confirmed MDRO † | 7 (10.3%) | 5 (8.5%) | 0.96 |
| >1 clinical indication noted | 22 (32.4%) | 30 (50.8%) | 0.05 |
| No indication specified | 7 (10.3%) | 5 (8.5%) | 0.96 |
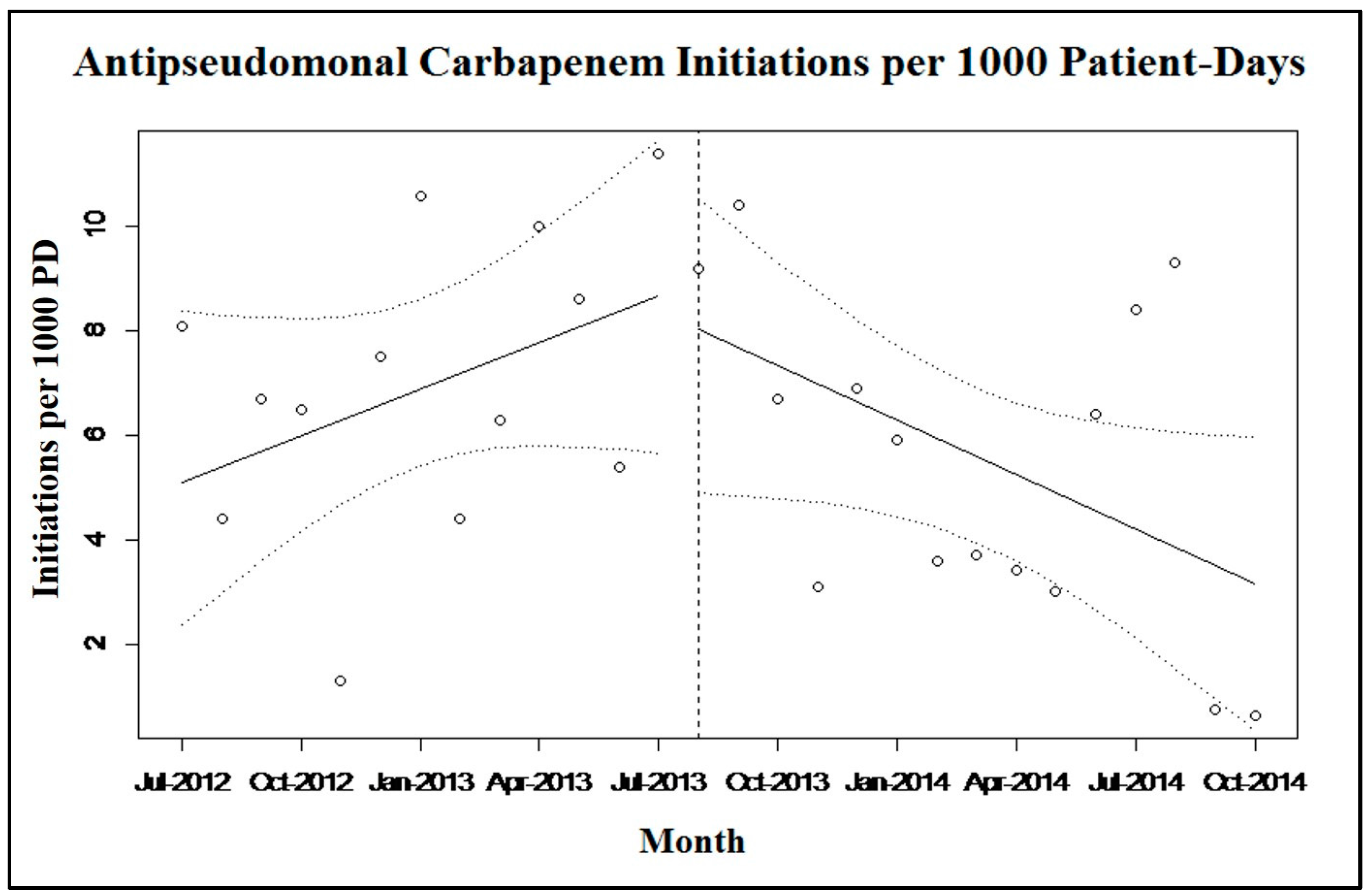
2.2. Discussion
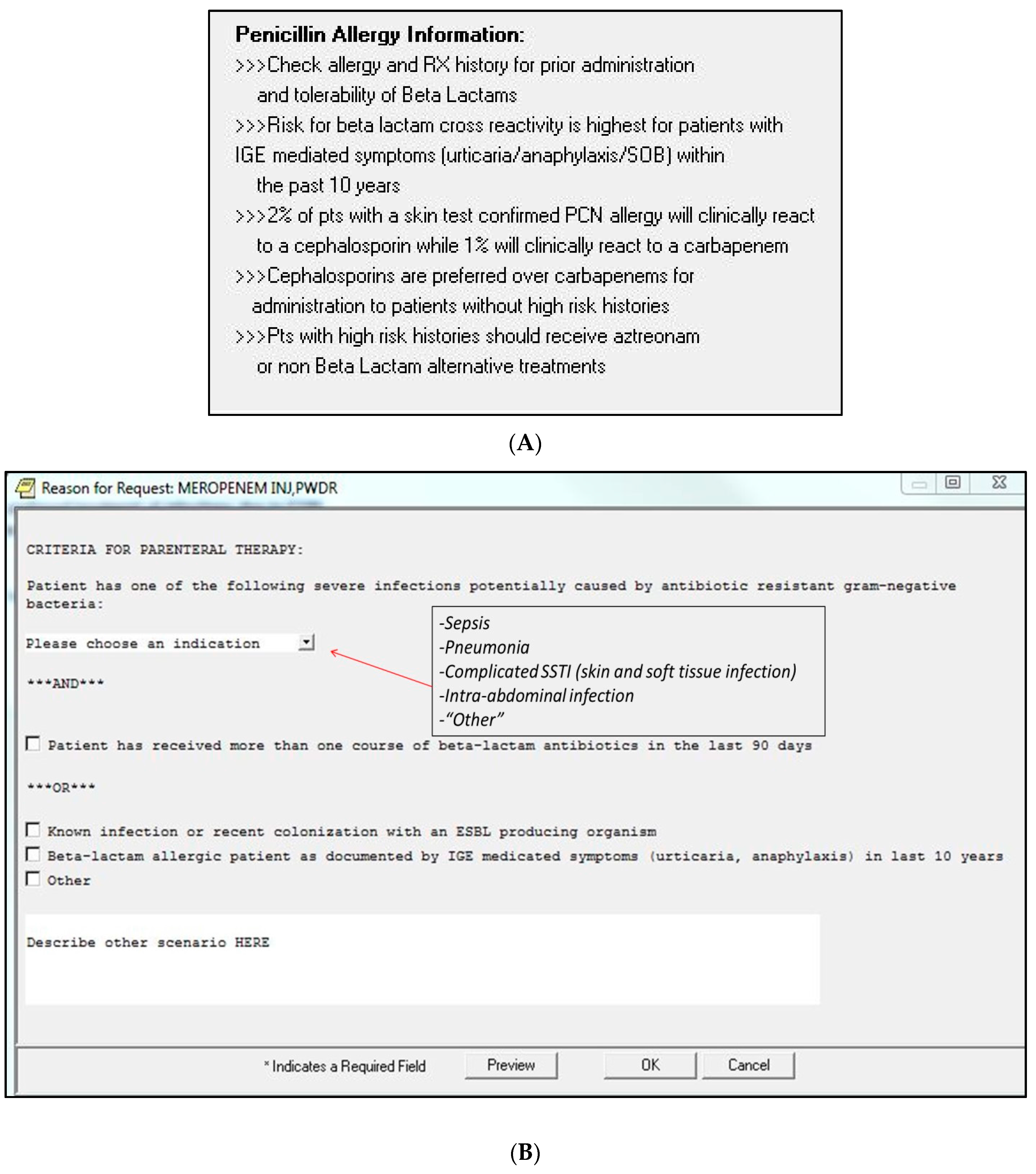
3. Materials and Methods
3.1. Design
3.2. Intervention
3.3. Data Collection
3.4. Definitions
3.5. Outcomes
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanj, S.S.; Kanafani, Z.A. Current concepts in antimicrobial therapy against resistant gram-negative organisms: Extended-spectrum β-lactamase-producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Pseudomonas aeruginosa. Mayo Clin. Proc. 2011, 86, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.N.; Moellering, R.C.; Eliopoulos, G.M.; Chambers, H.F.; Saag, M.S. The Sanford Guide to Antimicrobial Therapy 2013; Antimicrobial Therapy, Inc.: Sperryville, VA, USA, 2013. [Google Scholar]
- Pakyz, A.L.; Macdougall, C.; Oinonen, M.; Polk, R.E. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern. Med. 2008, 168, 2254–2260. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2013. Available online: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf (accessed on 4 May 2015).
- Falagas, M.E.; Tansarli, G.S.; Karageorgopoulos, D.E.; Vardakas, K.Z. Deaths attributable to carbapenem-resistant Enterobacteriaceae infections. Emerg. Infect. Dis. 2014, 20, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Neidell, M.J.; Cohen, B.; Furuya, Y.; Hill, J.; Jeon, C.Y.; Glied, S.; Larson, E.L. Costs of healthcare- and community-associated infections with antimicrobial-resistant versus antimicrobial-susceptible organisms. Clin. Infect. Dis. 2012, 55, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2012 CRE Toolkit-Guidance for Control of Carbapenem-resistant Enterobacteraciae. CDC: Atlanta, GA, USA, 2012. Available online: http://www.cdc.gov/hai/organisms/cre/cre-toolkit/index.html (accessed on 6 May 2015). [Google Scholar]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [PubMed]
- Forrest, G.N.; van Schooneveld, T.C.; Kullar, R.; Schulz, L.T.; Duong, P.; Postelnick, M. Use of electronic health records and clinical decision support systems for antimicrobial stewardship. Clin. Infect. Dis. 2014, 59, S122–S133. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.; Park, M.A. Penicillin skin testing in the evaluation and management of penicillin allergy. Ann. Allergy Asthma Immunol. 2011, 106. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar]
- Torres, M.J.; Blanca, M. The complex clinical picture of beta-lactam hypersensitivity: Penicillins, cephalosporins, monobactams, carbapenems, and clavams. Med. Clin. North Am. 2010, 94, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Pre-Pen [Package Insert]; AllerQuest: Plainville, CT, USA, 2013.
- Gonzalez-estrada, A.; Radojicic, C. Penicillin allergy: A practical guide for clinicians. Cleve Clin. J. Med. 2015, 82, 295–300. [Google Scholar] [PubMed]
- Romano, A.; Viola, M.; Guéant-rodriguez, R.M.; Gaeta, F.; Valluzzi, R.; Guéant, J.L. Brief communication: Tolerability of meropenem in patients with IgE-mediated hypersensitivity to penicillins. Ann. Intern. Med. 2007, 146, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.C.; Peters, L.; Leaders, C.B.; Wille, J.A. Pharmacist-managed service providing penicillin allergy skin tests. Am. J. Health Syst. Pharm. 2004, 61, 1271–1275. [Google Scholar] [PubMed]
- Addison, J.; Whitcombe, J.; William Glover, S. How doctors make use of online, point-of-care clinical decision support systems: A case study of UpToDate©. Health Inf. Libr. J. 2013, 30, 13–22. [Google Scholar] [CrossRef] [PubMed]
- UpToDate. Editorial Policy. 2014. Available online: http://www.uptodate.com/home/editorial-policy (accessed on 27 April 2015).
- Abbo, L.M.; Cosgrove, S.E.; Pottinger, P.S.; Pereyra, M.; Sinkowitz-Cochran, R.; Srinivasan, A.; Webb, D.J.; Hooton, T.M. Medical students’ perceptions and knowledge about antimicrobial stewardship: How are we educating our future prescribers? Clin. Infect. Dis. 2013, 57, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Solensky, R. Penicillin-Allergic Patients: Use of Cephalosporins, Carbapenems, and Monobactams. UpToDate. Available online: http://www.uptodate.com/contents/penicillin-allergic-patients-use-of-cephalosporins-carbapenems-and-monobactams (accessed on 18 March 2015).
- Unger, N.R.; Gauthier, T.P.; Cheung, L.W. Penicillin skin testing: Potential implications for antimicrobial stewardship. Pharmacotherapy 2013, 33, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Frumin, J.; Gallagher, J.C. Allergic cross-sensitivity between penicillin, carbapenem, and monobactam antibiotics: What are the chances? Ann. Pharmacother. 2009, 43, 304–315. [Google Scholar] [PubMed]
- Dudley, M.N.; Ambrose, P.G.; Bhavnani, S.M.; Craig, W.A.; Ferraro, M.J.; Jones, R.N.; Antimicrobial Susceptibility Testing Subcommittee of the Clinical and Laboratory Standards Institute. Background and rationale for revised clinical and laboratory standards institute interpretive criteria (Breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. Cephalosporins and Aztreonam. Clin. Infect. Dis. 2013, 56, 1301–1309. [Google Scholar] [PubMed]
- National Center for Health Statistics. Health, United States, 2014: With Special Feature on Adults Aged 55–64; Centers for Disease Control and Prevention: Hyattsville, MD, USA, 2015. Available online: http://www.cdc.gov/nchs/data/hus/hus14.pdf (accessed on 6 May 2015).
- Pakyz, A.L.; Oinonen, M.; Polk, R.E. Relationship of carbapenem restriction in 22 university teaching hospitals to carbapenem use and carbapenem-resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2009, 53, 1983–1986. [Google Scholar] [CrossRef] [PubMed]
- Kaki, R.; Elligsen, M.; Walker, S.; Simor, A.; Palmay, L.; Daneman, N. Impact of antimicrobial stewardship in critical care: A systematic review. J. Antimicrob. Chemother. 2011, 66, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, Y.; Troillet, N.; Eliopoulos, G.M.; Samore, M.H. Emergence of antibiotic-resistant Pseudomonas aeruginosa: Comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother. 1999, 43, 1379–1382. [Google Scholar] [PubMed]
- Wagner, A.K.; Soumerai, S.B.; Zhang, F.; Ross-Degnan, D. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 2002, 27, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Blanca, M.; Vega, J.M.; Moreno, F.; Carmona, M.J.; García, J.J.; Segurado, E.; Justicia, J.L.; Juarez, C. Cross-reactivity between a penicillin and a cephalosporin with the same side chain. J. Allergy Clin. Immunol. 1996, 98, 671–677. [Google Scholar] [CrossRef]
- Davey, P.; Brown, E.; Charani, E.; Fenelon, L.; Gould, I.M.; Holmes, A.; Ramsay, C.R.; Wiffen, P.J.; Wilcox, M. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2013, 30. [Google Scholar] [CrossRef]
- Kullar, R.; Goff, D.A. Transformation of antimicrobial stewardship programs through technology and informatics. Infect. Dis. Clin. North Am. 2014, 28, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.D.; Bradham, D.D.; Baumgarten, M.; Zuckerman, I.H.; Fink, J.C.; Perencevich, E.N. The use and interpretation of quasi-experimental studies in infectious diseases. Clin. Infect. Dis. 2004, 38, 1586–1591. [Google Scholar] [PubMed]
- Rimawi, R.H.; Cook, P.P.; Gooch, M.; Kabchi, B.; Ashraf, M.S.; Rimawi, B.H.; Gebregziabher, M.; Siraj, D.S. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J. Hosp. Med. 2013, 8, 341–345. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caplinger, C.; Smith, G.; Remington, R.; Madaras-Kelly, K. Evaluation of a Computerized Decision Support Intervention to Decrease Use of Anti-Pseudomonal Carbapenems in Penicillin Allergic Patients. Antibiotics 2016, 5, 7. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics5010007
Caplinger C, Smith G, Remington R, Madaras-Kelly K. Evaluation of a Computerized Decision Support Intervention to Decrease Use of Anti-Pseudomonal Carbapenems in Penicillin Allergic Patients. Antibiotics. 2016; 5(1):7. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics5010007
Chicago/Turabian StyleCaplinger, Christina, Garret Smith, Richard Remington, and Karl Madaras-Kelly. 2016. "Evaluation of a Computerized Decision Support Intervention to Decrease Use of Anti-Pseudomonal Carbapenems in Penicillin Allergic Patients" Antibiotics 5, no. 1: 7. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics5010007





