Identification of FDA-Approved Drugs with Activity against Stationary Phase Bartonella henselae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain, Culture Media, and Culture Conditions
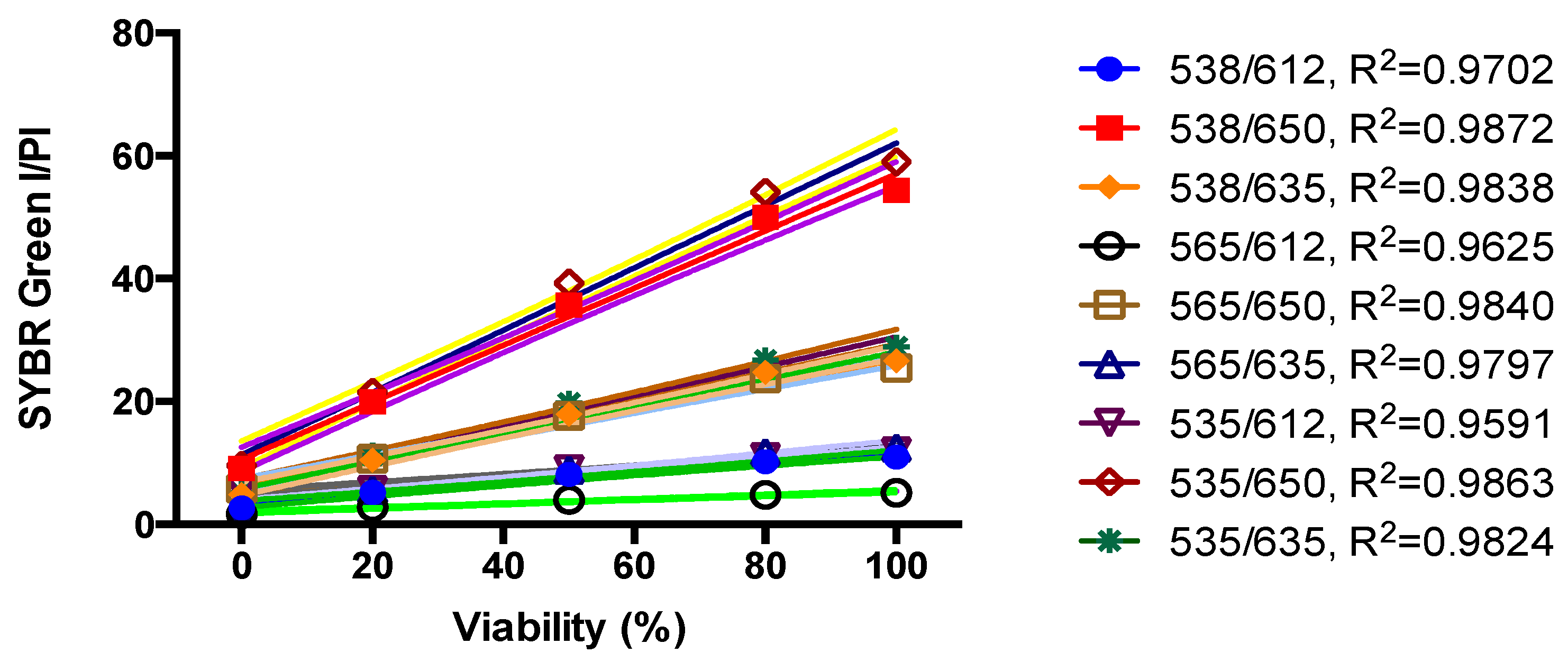
2.2. Standard Curve of SYBR Green I/PI Assay for B. henselae
2.3. Antibiotics and the FDA Drug Library
2.4. Microscopy Techniques
2.5. Screening of FDA-Approved Drug Library against Stationary Phase B. henselae
2.6. Drug Exposure Assay
2.7. Minimum inhibitory concentration (MIC) Determination
3. Results
3.1. Growth Behavior of B. henselae in Modified Schneider’s Medium
3.2. Development of a SYBR Green/PI Viability Assay for B. henselae
3.3. Screening FDA Drug Library to Identify Drugs Active against Non-Growing Stationary Phase B. henselae Using the SYBR Green/PI Assay
3.4. MIC Determination of Active Hits
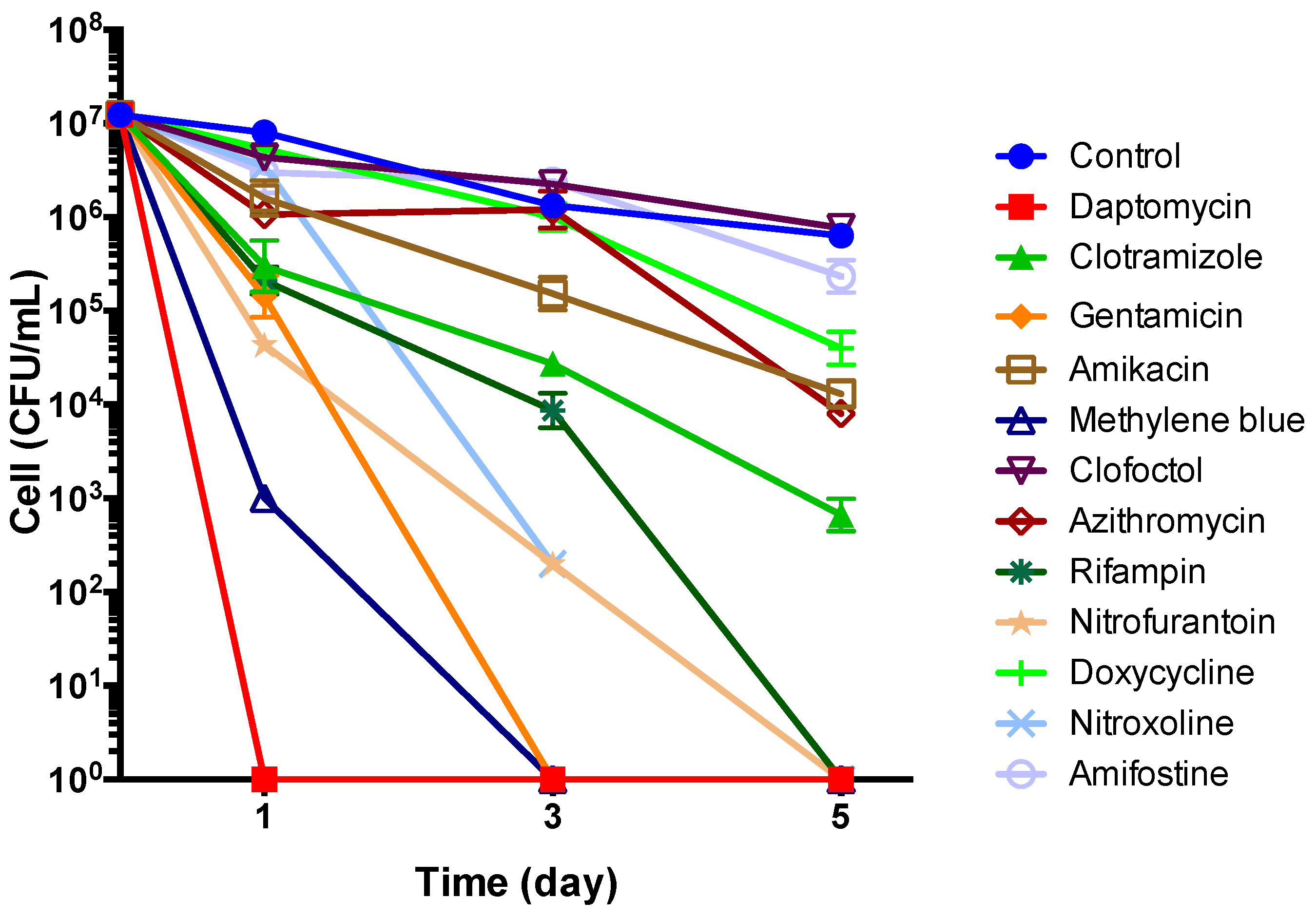
3.5. Subculture Study of Stationary Phase B. henselae After Drug Exposure
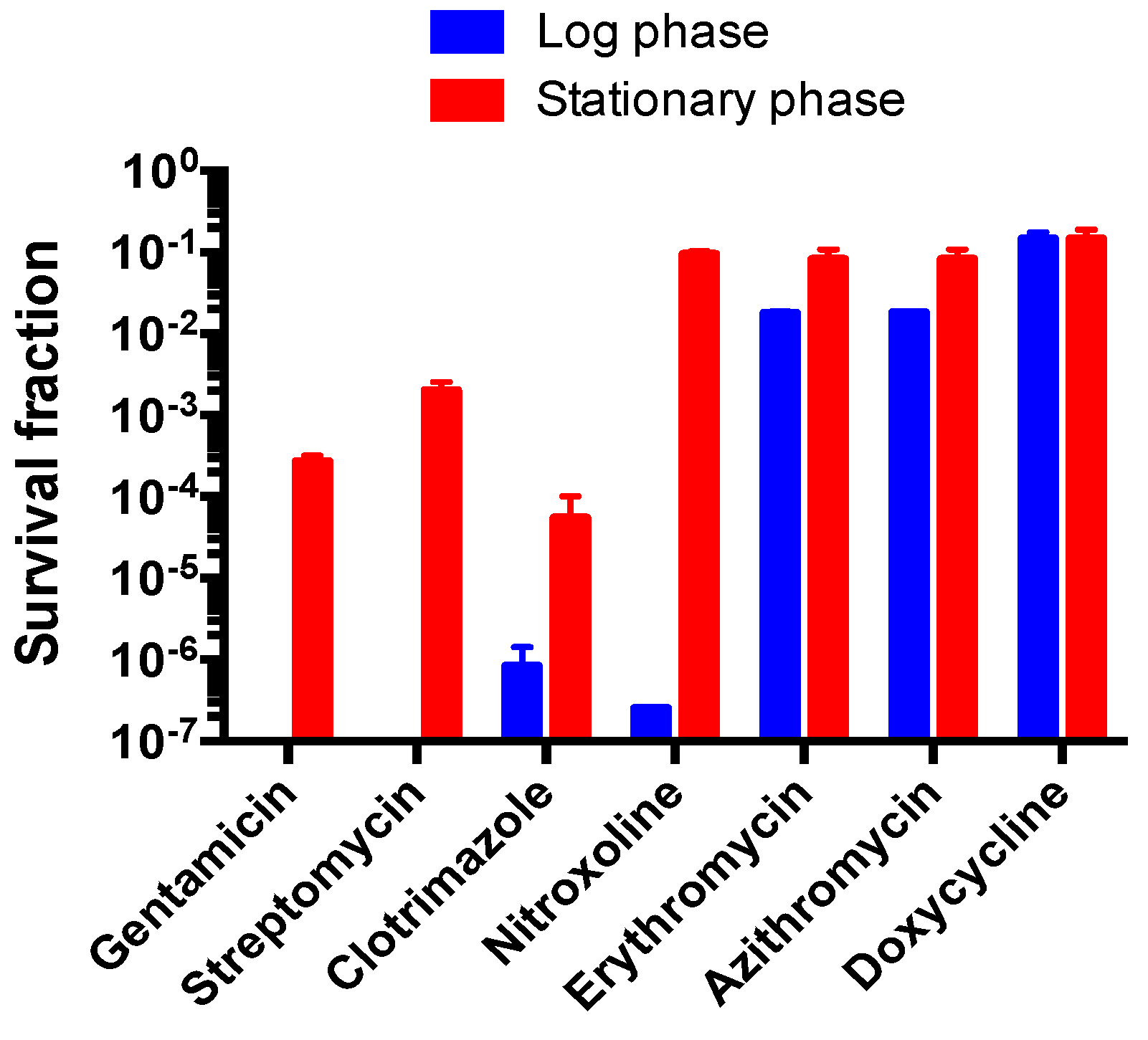
3.6. Comparison of Susceptibility of Log Phase B. henselae and Stationary Phase B. henselae in Drug Exposure Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okaro, U.; Addisu, A.; Casanas, B.; Anderson, B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin. Microbiol. Rev. 2017, 30, 709–746. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B. Bartonellosis, One Health and all creatures great and small. Vet. Dermatol. 2017, 28, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Jacomo, V.; Kelly, P.; Raoult, D. Natural history of Bartonella infections (an exception to Koch’s postulate). Clin. Diagn. Lab. Immunol. 2002, 9, 8–18. [Google Scholar] [CrossRef]
- Rolain, J.; La Scola, B.; Liang, Z.; Davoust, B.; Raoult, D. Immunofluorescent Detection of IntraerythrocyticBartonella henselae in Naturally Infected Cats. J. Clin. Microbiol. 2001, 39, 2978–2980. [Google Scholar] [CrossRef] [PubMed]
- Mosepele, M.; Mazo, D.; Cohn, J. Bartonella infection in immunocompromised hosts: Immunology of vascular infection and vasoproliferation. Clin. Dev. Immunol. 2012. [Google Scholar] [CrossRef]
- Eskow, E.; Rao, R.-V.S.; Mordechai, E. Concurrent infection of the central nervous system by Borrelia burgdorferi and Bartonella henselae: Evidence for a novel tick-borne disease complex. Arch. Neurol. 2001, 58, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Chomel, B.B.; Boulouis, H.-J.; Maruyama, S.; Breitschwerdt, E.B. Bartonella spp. in pets and effect on human health. Emerg. Infect. Dis. 2006, 12, 389. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Raoult, D. Pathogenicity and treatment of Bartonella infections. Int. J. Antimicrob. Agents 2014, 44, 16–25. [Google Scholar] [CrossRef]
- Biswas, S.; Rolain, J.-M. Bartonella infection: Treatment and drug resistance. Future Microbiol. 2010, 5, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.W.; Vincent, J.M.; Person, D.A. The expanding spectrum of Bartonella infections: II. Cat-scratch disease. Pediatr. Infect. Dis. J. 1997, 16, 163–179. [Google Scholar] [CrossRef]
- Zhang, Y. Persisters, Persistent Infections and the Yin-Yang Model. Emerg. Microb. Infect. 2014, 3, e3. [Google Scholar] [CrossRef]
- Feng, J.; Wang, T.; Zhang, S.; Shi, W.; Zhang, Y. An optimized SYBR Green I/PI assay for rapid viability assessment and antibiotic susceptibility testing for Borrelia burgdorferi. PLoS ONE 2014, 9, e111809. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of Novel Activity against Borrelia burgdorferi Persisters Using an FDA Approved Drug Library. Emerg. Microb. Infect. 2014, 3, e49. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Weitner, M.; Shi, W.; Zhang, S.; Sullivan, D.; Zhang, Y. Identification of Additional Anti-Persister Activity against Borrelia burgdorferi from an FDA Drug Library. Antibiotics 2015, 4, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. A Drug Combination Screen Identifies Drugs Active against Amoxicillin-Induced Round Bodies of In Vitro Borrelia burgdorferi Persisters from an FDA Drug Library. Front. Microbiol. 2016, 7, 743. [Google Scholar] [CrossRef]
- Lynch, T.; Iverson, J.; Kosoy, M. Combining culture techniques for Bartonella: The best of both worlds. J. Clin. Microbiol. 2011, 49, 1363–1368. [Google Scholar] [CrossRef] [PubMed]
- Riess, T.; Dietrich, F.; Schmidt, K.V.; Kaiser, P.O.; Schwarz, H.; Schäfer, A.; Kempf, V.A. Analysis of a Novel Insect Cell Culture Medium-Based Growth Medium for Bartonella Species. Appl. Environ. Microbiol. 2008, 74, 5224–5227. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 13 December 2018).
- Rolain, J.; Brouqui, P.; Koehler, J.; Maguina, C.; Dolan, M.; Raoult, D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob. Agents Chemother. 2004, 48, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Ruslami, R.; Nijland, H.M.; Alisjahbana, B.; Parwati, I.; van Crevel, R.; Aarnoutse, R.E. Pharmacokinetics and tolerability of a higher rifampin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob. Agents Chemother. 2007, 51, 2546–2551. [Google Scholar] [CrossRef]
- Matzneller, P.; Krasniqi, S.; Kinzig, M.; Sörgel, F.; Hüttner, S.; Lackner, E.; Müller, M.; Zeitlinger, M. Blood, tissue and intracellular concentrations of Azithromycin during and after end of therapy. Antimicrob. Agents Chemother. 2013, 57, 1736–1742. [Google Scholar] [CrossRef]
- Smith, T.; Kinkei, A.; Gryczko, C.; Goulet, J. Absorption of pyrvinium pamoate. Clin. Pharmacol. Therap. 1976, 19, 802–806. [Google Scholar] [CrossRef]
- Walter-Sack, I.; Rengelshausen, J.; Oberwittler, H.; Burhenne, J.; Mueller, O.; Meissner, P.; Mikus, G. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur. J. Clin. Pharmacol. 2009, 65, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Welling, P.G.; Koch, P.A.; Lau, C.C.; Craig, W.A. Bioavailability of tetracycline and doxycycline in fasted and nonfasted subjects. Antimicrob. Agents Chemother. 1977, 11, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Geerdes-Fenge, H.; Goetschi, B.; Rau, M.; Borner, K.; Koeppe, P.; Wettich, K.; Lode, H. Comparative pharmacokinetics of dirithromycin and erythromycin in normal volunteers with special regard to accumulation in polymorphonuclear leukocytes and in saliva. Eur. J. Clin. Pharmacol. 1997, 53, 127–133. [Google Scholar] [CrossRef]
- Randinitis, E.J.; Brodfuehrer, J.I.; Eiseman, I.; Vassos, A.B. Pharmacokinetics of clinafloxacin after single and multiple doses. Antimicrob. Agents Chemother. 2001, 45, 2529–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novelli, A.; Rosi, E. Pharmacological properties of oral antibiotics for the treatment of uncomplicated urinary tract infections. J. Chemother. 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Bergogne-Berezin, E.; Berthelot, G.; Muller-Serieys, C. Present status of nitroxoline. Pathol. Biol. (Paris) 1987, 35, 873–878. [Google Scholar] [PubMed]
- Girard, P.-M.; Clair, B.; Certain, A.; Bidault, R.; Matheron, S.; Regnier, B.; Farinotti, R. Comparison of plasma concentrations of aerosolized pentamidine in nonventilated and ventilated patients with pneumocystosis. Am. Rev. Respir. Dis. 1989, 140, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Plempel, M. Pharmacokinetics of imidazole antimycotics. Postgrad Med. J. 1979, 55, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Demczar, D.J.; Nafziger, A.N.; Bertino, J. Pharmacokinetics of gentamicin at traditional versus high doses: Implications for once-daily aminoglycoside dosing. Antimicrob. Agents Chemother. 1997, 41, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Ding, L.; Chen, Y.; Gong, B.; He, J.; Xu, G. Determination of berberine in human plasma by liquid chromatography–electrospray ionization–mass spectrometry. J. Pharma Biomed. Anal. 2007, 44, 931–937. [Google Scholar] [CrossRef]
- Kays, M.B.; Overholser, B.R.; Mueller, B.A.; Moe, S.M.; Sowinski, K.M. Effects of sevelamer hydrochloride and calcium acetate on the oral bioavailability of ciprofloxacin. Am. J. Kidney Dis. 2003, 42, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Shen, J.-Y.; Yin, W.-L.; Yao, J.-Y.; Xu, Y.; Pan, X.-Y.; Hao, G.-J. Pharmacokinetic Disposition of Streptomycin Sulfate in Japanese Eel (Anguilla japonica) after Oral and Intramuscular Administrations. Pharmacol. Pharma. 2012, 3, 195. [Google Scholar] [CrossRef]
- Mikamo, H.; Kawazoe, K.; Sato, Y.; Ito, K.; Tamaya, T. Pharmacokinetics of miconazole in serum and exudate of pelvic retroperitoneal space after radical hysterectomy and pelvic lymphadenectomy. Int. J. Antimicrob. Agents 1998, 9, 207–211. [Google Scholar] [CrossRef]
- White, B.P.; Lomaestro, B.; Pai, M.P. Optimizing the initial amikacin dosage in adults. Antimicrob. Agents Chemother. 2015, 59, 7094–7096. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nakamura, Y.; Tsuya, A.; Murakami, H.; Endo, M.; Yamamoto, N. Pharmacokinetics of aprepitant and dexamethasone after administration of chemotherapeutic agents and effects of plasma substance P concentration on chemotherapy-induced nausea and vomiting in Japanese cancer patients. Cancer Chemother. Pharmacol. 2011, 68, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Dvorchik, B.H.; Brazier, D.; DeBruin, M.F.; Arbeit, R.D. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 2003, 47, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Danesi, R.; Gasperini, M.; Senesi, S.; Freer, G.; Angeletti, C.; Del, M.T. A pharmacokinetic study of clofoctol in human plasma and lung tissue by using a microbiological assay. Drugs Exp. Clin. Res. 1988, 14, 39–43. [Google Scholar] [PubMed]
- Koomanachai, P.; Landersdorfer, C.B.; Chen, G.; Lee, H.J.; Jitmuang, A.; Wasuwattakul, S.; Sritippayawan, S.; Li, J.; Nation, R.L.; Thamlikitkul, V. Pharmacokinetics of colistin methanesulfonate and formed colistin in end-stage renal disease patients receiving continuous ambulatory peritoneal dialysis. Antimicrob. Agents Chemother. 2014, 58, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Souid, A.-K.; Dubowy, R.L.; Blaney, S.M.; Hershon, L.; Sullivan, J.; McLeod, W.D.; Bernstein, M.L. Phase I clinical and pharmacologic study of weekly cisplatin and irinotecan combined with amifostine for refractory solid tumors. Clin. Cancer Res. 2003, 9, 703–710. [Google Scholar]
- Dörbecker, C.; Sander, A.; Oberle, K.; Schülin-Casonato, T. In vitro susceptibility of Bartonella species to 17 antimicrobial compounds: Comparison of Etest and agar dilution. J. Antimicrob. Chemother. 2006, 58, 784–788. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Kordick, D.L. Bartonella infection in animals: Carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 2000, 13, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Rolain, J.-M.; Maurin, M.; Mallet, M.-N.; Parzy, D.; Raoult, D. Culture and antibiotic susceptibility of Bartonella quintana in human erythrocytes. Antimicrob. Agents Chemother. 2003, 47, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. Lest we forget you—methylene blue. Neurobiol. Aging 2011, 32, 2325. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Fatima, Z.; Hameed, S. Antifungal Action of Methylene Blue Involves Mitochondrial Dysfunction and Disruption of Redox and Membrane Homeostasis in C. albicans. Open Microbiol. J. 2016, 10, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug Combinations against Borrelia burgdorferi Persisters In Vitro: Eradication Achieved by Using Daptomycin, Cefoperazone and Doxycycline. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Perlmutter, N.G.; Shapiro, H.M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.P.; Mariner, K.R.; Chopra, I.; O’Neill, A.J. The target of daptomycin is absent from Escherichia coli and other gram-negative pathogens. Antimicrob. Agents Chemother. 2013, 57, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Kadavakollu, S.; Stailey, C.; Kunapareddy, C.; White, S. Clotrimazole as a cancer drug: A short review. Med. Chem. 2014, 4, 722. [Google Scholar]
- Huy, N.T.; Takano, R.; Hara, S.; Kamei, K. Enhancement of heme-induced membrane damage by the anti-malarial clotrimazole: The role of colloid-osmotic forces. Biol. Pharmacol. Bull. 2004, 27, 361–365. [Google Scholar] [CrossRef]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Pulliainen, A.T.; Dehio, C. Persistence of Bartonella spp. stealth pathogens: From subclinical infections to vasoproliferative tumor formation. FEMS Microbiol. Rev. 2012, 36, 563–599. [Google Scholar] [CrossRef] [PubMed]


| Drugs (50 μM) | Residual Viable Cell Percentage | |
|---|---|---|
| Primary Screen b | Confirmation Microscopy c | |
| Drug free control | 60% | 81% |
| Gentamicin | 2% | 32% |
| Azithromycin | 17% | 42% |
| Streptomycin | 9% | 39% |
| Tetracycline | 12% | 50% |
| Rifampin | 2% | 59% |
| Doxycycline | 11% | 60% |
| Penicillin | 13% | 60% |
| Erythromycin | 14% | 66% |
| Chloramphenicol | 13% | 70% |
| Cefuroxime | 21% | 73% |
| Ciprofloxacin | 30% | 74% |
| Pyrvinium pamoate | 0% d | ND e |
| Amifostine | 27% | 10% |
| Daptomycin | 7% | 21% |
| Lopinavir/ritonavir | 9% | 21% |
| Sulconazole | 3% | 22% |
| Econazole | 8% | 22% |
| Oxiconazole | 3% | 24% |
| Butoconazole | 11% | 25% |
| Methylene blue | 16% | 25% |
| Clotrimazole | 7% | 27% |
| Dibekacin * | 9% | 28% |
| Abamectin * | 2% | 30% |
| Colistin | 4% | 30% |
| Bifonazole | 4% | 31% |
| Amikacin | 12% | 32% |
| Nitroxoline | 6% | 33% |
| Miconazole | 8% | 35% |
| Chlorosalicylanilide * | 17% | 38% |
| Berberine | 8% | 39% |
| Meclocycline * | 11% | 40% |
| Nebramycin * | 2% | 41% |
| Kanamycin | 9% | 44% |
| Dichlorophen * | 14% | 45% |
| Verteporfin | 0% d | 45% |
| Pentamidine | 15% | 46% |
| Cloxyquin * | 7% | 48% |
| Aprepitant | 0% d | 49% |
| Puromycin * | 6% | 49% |
| Amaranth * | 3% | 50% |
| Lomerizine * | 8% | 51% |
| Carbomycin * | 16% | 52% |
| Spiramycin * | 14% | 53% |
| Thiethylperazine | 4% | 55% |
| Clinafloxacin | 11% | 56% |
| Clofoctol | 12% | 58% |
| Meclizine | 11% | 60% |
| Pazufloxacin * | 12% | 62% |
| Nitrofurantoin | 16% | 63% |
| Diclazuril * | 15% | 64% |
| Olsalazine | 18% | 64% |
| Nifuroxazide * | 7% | 65% |
| Antibiotics | MIC (μg/mL) | Cmax (μg/mL) |
|---|---|---|
| Rifampin | 0.01 | 15.6 [20] |
| Azithromycin | 0.04–0.08 | 0.57 ± 0.23 [21] |
| Pyrvinium pamoate | 0.04–0.08 | 0.003 * [22] |
| Methylene blue | 0.08–0.16 | 3.91 ± 1.60 [23] |
| Doxycycline | 0.08–0.16 | 1.5–7.0 [24] |
| Erythromycin | 0.08–0.16 | 1.44 [25] |
| Clinafloxacin | 0.16–0.31 | 5.0 [26] |
| Nitrofurantoin | 0.31–0.63 | 0.88–0.96 [27] |
| Nitroxoline | 0.31–0.63 | 5.59 ± 3.15 [28] |
| Pentamidine | 0.31–0.63 | 0.22 ± 0.05 [29] |
| Clotrimazole | 0.63–1.25 | 0.5–1.5 [30] |
| Gentamicin | 0.63–1.25 | 11.0 ± 0.6 [31] |
| Berberine | 0.63–1.25 | 0.00044 ± 0.00041 [32] |
| Ciprofloxacin | 1.25–2.5 | 1.97–5.39 [33] |
| Streptomycin | 3.13–6.25 | 29.52 [34] |
| Miconazole | 3.13–6.25 | 6.26 [35] |
| Amikacin | 6.25–12.5 | 101 ± 49.4 [36] |
| Aprepitant | 10–20 | 3.07 ± 0.85 [37] |
| Daptomycin | 12.5–25 | 55–133 [38] |
| Clofoctol | 70–140 | 38.1 [39] |
| Colistin | 80 | 1.21–3.36 [40] |
| Amifostine | 160 | 16.99–19.89 [41] |
| Antimicrobial Agents | Con. of Drug Exposure (μg/mL) | CFU per mL after Drug Exposure | |
|---|---|---|---|
| 1 Day | 3 Day | ||
| Control * | 0 | 3.67 ± 2.08 × 107 | 1.33 ± 0.11 × 106 |
| Rifampin | 10 | 2.10 ± 0.85 × 105 | 8.67 ± 0.46 × 103 |
| Azithromycin | 2 | 3.00 ± 1.00 × 106 | 5.33 ± 2.31 × 105 |
| Doxycycline | 5 | 5.33 ± 1.53 × 106 | 1.00 ± 0.40 × 106 |
| Erythromycin | 1 | 3.00 ± 1.00 × 106 | 1.00 ± 0.20 × 106 |
| Ciprofloxacin | 5 | 1.77 ± 0.45 × 106 | 2.60 ± 1.40 × 105 |
| Gentamicin | 10 | 1.00 ± 0.17 × 104 | 0 |
| Streptomycin | 25 | 7.33 ± 2.08 × 104 | 0 |
| Amikacin | 100 | 2.00 ± 1.73 × 103 | 0 |
| Methylene blue | 5 | 0 | 0 |
| Daptomycin | 60 | 0 | 0 |
| Pyrvinium pamoate | 5 | 0 | 0 |
| Clotrimazole | 25 | 2.00 ± 1.73 × 103 | 0 |
| Nitroxoline | 5 | 3.47 ± 0.31 × 106 | 2.00 ± 0.00 × 102 |
| Nitrofurantoin | 1 | 3.00 ± 0.00 × 105 | 9.33 ± 1.15 × 103 |
| Clinafloxacin | 5 | 9.00 ± 1.00 × 105 | 5.33 ± 3.06 × 104 |
| Clofoctol | 35 | 2.20 ± 0.72 × 106 | 1.00 ± 0.53 × 105 |
| Miconazole | 6 | 2.07 ± 0.38 × 106 | 2.13 ± 0.31 × 105 |
| Pentamidine | 0.5 | 2.00 ± 1.00 × 106 | 2.00 ± 0.00 × 105 |
| Aprepitant | 2 | 1.20 ± 0.17 × 107 | 9.00 ± 2.65 × 105 |
| Colistin | 2 | 7.33 ± 1.15 × 106 | 6.67 ± 3.06 × 105 |
| Amifostine | 15 | 3.00 ± 1.00 × 106 | 5.33 ± 2.23 × 105 |
| Berberine | 1 | 3.40 ± 0.27 × 106 | 1.00 ± 0.00 × 106 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Feng, J.; Xiao, S.; Shi, W.; Sullivan, D.; Zhang, Y. Identification of FDA-Approved Drugs with Activity against Stationary Phase Bartonella henselae. Antibiotics 2019, 8, 50. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8020050
Li T, Feng J, Xiao S, Shi W, Sullivan D, Zhang Y. Identification of FDA-Approved Drugs with Activity against Stationary Phase Bartonella henselae. Antibiotics. 2019; 8(2):50. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8020050
Chicago/Turabian StyleLi, Tingting, Jie Feng, Shuzhen Xiao, Wanliang Shi, David Sullivan, and Ying Zhang. 2019. "Identification of FDA-Approved Drugs with Activity against Stationary Phase Bartonella henselae" Antibiotics 8, no. 2: 50. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8020050





