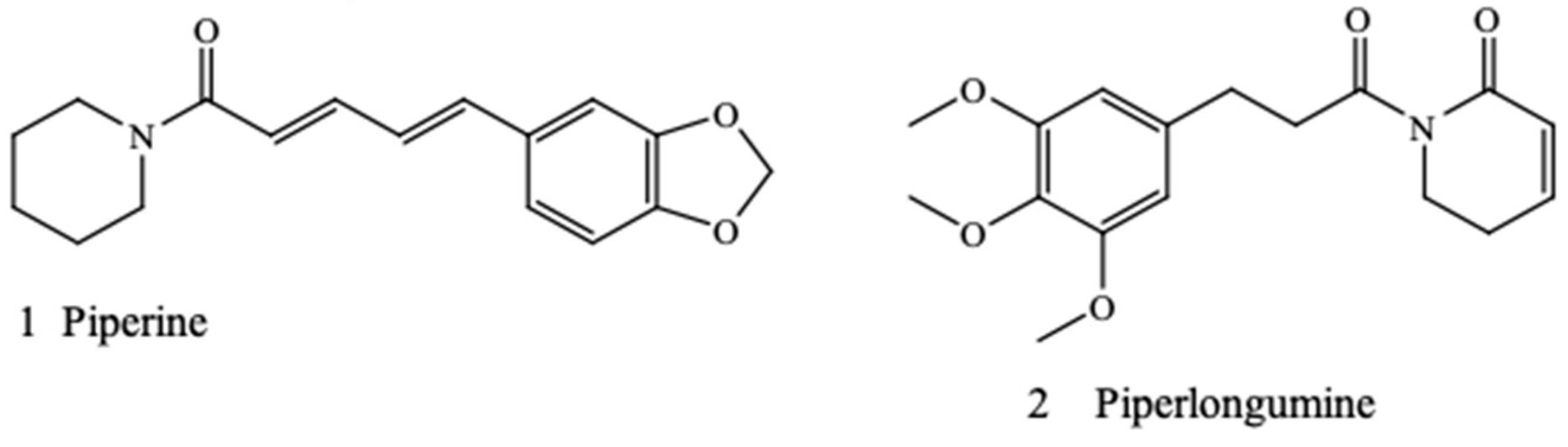
Antimicrobial and Synergistic Effects of Commercial Piperine and Piperlongumine in Combination with Conventional Antimicrobials
Abstract
:1. Introduction
2. Results
3. Discussion
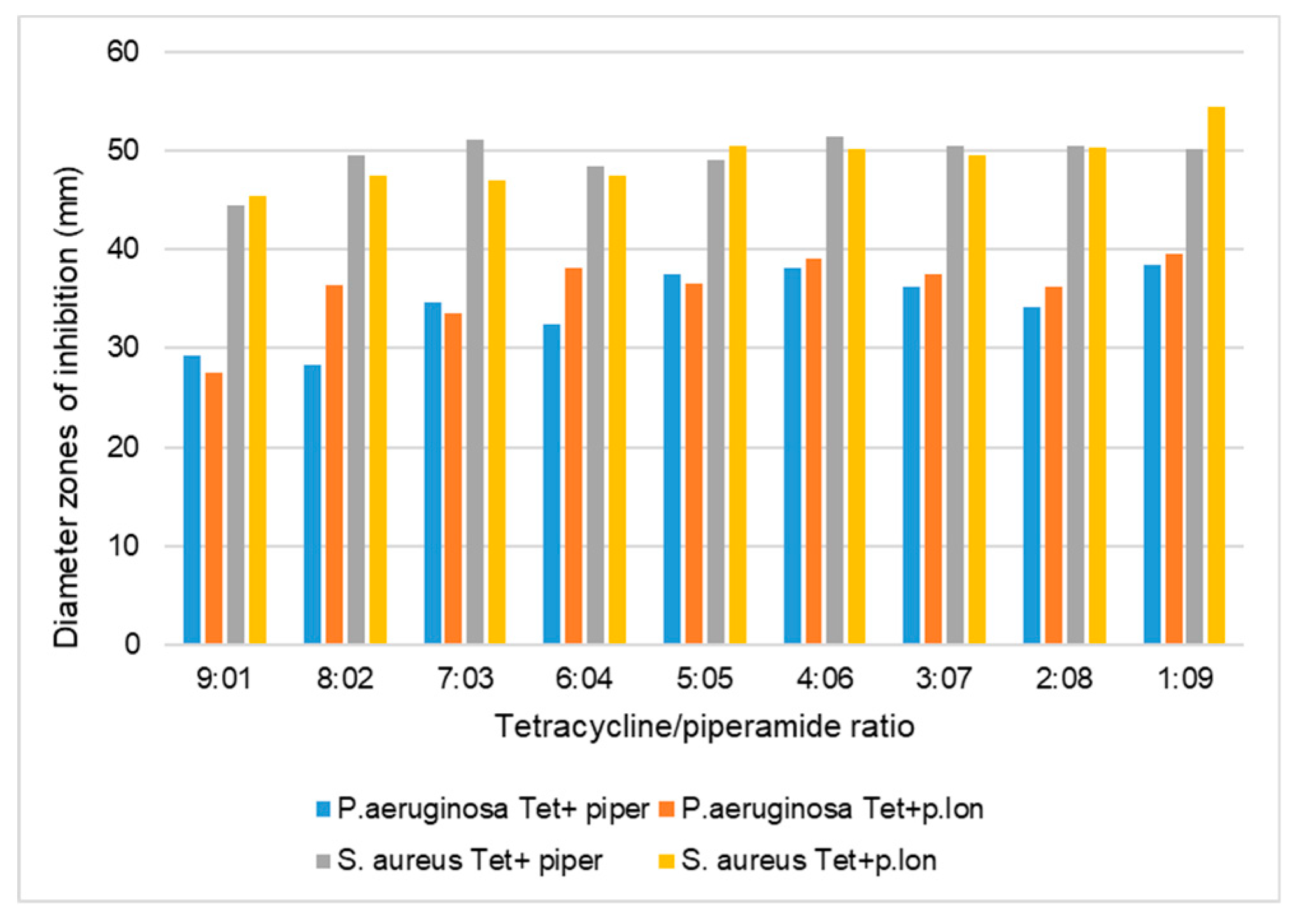
3.1. Antibacterial Synergistic Effects of Piperine and Piperlongumine against S. aureus
3.2. Antibacterial Synergistic Effects of Piperine and Piperlongumine against P. aeruginosa
3.3. Antifungal Synergistic Effects of Piperine and Piperlongumine against C. albicans
4. Materials and Methods
4.1. Sources of Antibiotics and Commercial Compounds
4.2. Preparation of Piperine, Piperlongumine, and Antimicrobials
4.3. Agar Disk-Diffusion Method
4.4. Microdilution Method for Minimum Inhibitory Concentration Estimation
4.5. Fractional Inhibitory Concentration Index
5. Conclusions
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brown, G.D.; Denning, D.W.; Gow, N.A.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden killers: human fungal infections. Sci. Transl. Med. 2012, 4, rv13–rv165. [Google Scholar] [CrossRef]
- Mulholland, E.K.; Adegbola, R.A. Bacterial infections--a major cause of death among children in Africa. N. Engl. J. Med. 2005, 352, 75–77. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Kourtesi, C.; Ball, A.R.; Huang, Y.Y.; Jachak, S.M.; Vera, D.M.A.; Khondkar, P.; Tegos, G.P. Suppl 1: Microbial efflux systems and inhibitors: approaches to drug discovery and the challenge of clinical implementation. Open Microbiol. J. 2013, 7, 34. [Google Scholar] [CrossRef]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech. 2017, 7, 172. [Google Scholar] [CrossRef]
- Alviano, D.S.; Alviano, C.S. Plant extracts: search for new alternatives to treat microbial diseases. Curr. Pharm. Biotechnol. 2009, 10, 106–121. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Kruthiventi, A.K.; Doble, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Lambert, R.J.W. Susceptibility testing: inoculum size dependency of inhibition using the Colworth MIC technique. J. Appl. Microbiol. 2000, 89, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Van Vuuren, S.F.; Suliman, S.; Viljoen, A.M. The antimicrobial activity of four commercial essential oils in combination with conventional antimicrobials. Lett. Appl. Microbiol. 2009, 48, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.K.; Low, L.Y.; Yap, P.S.X.; Yusoff, K.; Mai, C.W.; Lai, K.S.; Lim, S.H.E. Plant-derived antimicrobials: Insights into mitigation of antimicrobial resistance. Rec. Nat. Prod. 2018, 12, 295–316. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Yrjönen, T.; Vuorela, H.; Holm, Y. Bioactive compounds from medicinal plants: focus on Piper species. S. Afr. J. Bot. 2017, 112, 54–69. [Google Scholar] [CrossRef]
- Biavatti, M.W. Synergy: an old wisdom, a new paradigm for pharmacotherapy. Braz. J. Pharm. Sci. 2009, 45, 371–378. [Google Scholar] [CrossRef]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef]
- Piska, K.; Gunia-Krzyżak, A.; Koczurkiewicz, P.; Wójcik-Pszczoła, K.; Pękala, E. Piperlongumine (piplartine) as a lead compound for anticancer agents–Synthesis and properties of analogues. Eur. J. Med. Chem. 2018, 156, 13–20. [Google Scholar] [CrossRef]
- Naika, R.; Prasanna, K.P.; Ganapathy, P.S. Antibacterial activity of piperlongumine an alkaloid isolated from methanolic root extract of Piper Longum L. Pharmacophore. 2010, 1, 141–148. [Google Scholar]
- Karsha, P.V.; Lakshmi, O.B. Antibacterial activity of black pepper (Piper nigrum Linn.) with special reference to its mode of action on bacteria. Indian J. Nat. Prod. Resour. 2010, 1, 213–215. [Google Scholar]
- Scott, I.M.; Puniani, E.; Jensen, H.; Livesey, J.F.; Poveda, L.; Sánchez-Vindas, P.; Arnason, J.T. Analysis of Piperaceae germplasm by HPLC and LCMS: a method for isolating and identifying unsaturated amides from Piper spp extracts. J. Agric. Food Chem. 2005, 53, 1907–1913. [Google Scholar] [CrossRef]
- Adesina, S.K.; Adebayo, A.S.; Gröning, R. New constituents of Piper guineense fruit and leaf. Die Pharmazie. 2003, 58, 423–425. [Google Scholar] [CrossRef]
- Mirza, Z.M.; Kumar, A.; Kalia, N.P.; Zargar, A.; Khan, I.A. Piperine as an inhibitor of the MdeA efflux pump of Staphylococcus aureus. J. Med. Microbiol. 2011, 60, 1472–1478. [Google Scholar] [CrossRef]
- Stavri, M.; Piddock, L.J.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2006, 59, 1247–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philipova, I.; Valcheva, V.; Mihaylova, R.; Mateeva, M.; Doytchinova, I.; Stavrakov, G. Synthetic piperine amide analogs with antimycobacterial activity. Chem Biol. Drug Des. 2018, 91, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, D.; Silva, T.; Magalhães e Silva, D.; Remião, F.; Borges, F. Lessons from black pepper: piperine and derivatives thereof. Expert Opin. Ther. Pat. 2016, 26, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Umadevi, P.; Deepti, K.; Venugopal, D.V. Synthesis, anticancer and antibacterial activities of piperine analogs. Med. Chem. Res. 2013, 22, 5466–5471. [Google Scholar] [CrossRef]
- Dusane, D.H.; Hosseinidoust, Z.; Asadishad, B.; Tufenkji, N. Alkaloids modulate motility, biofilm formation and antibiotic susceptibility of uropathogenic Escherichia coli. PLoS ONE 2014, 9, e112093. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, H.Y.; Cha, J.D. Synergistic effects between silibinin and antibiotics on methicillin-resistant Staphylococcus aureus isolated from clinical specimens. Biotechnol. J. 2011, 6, 1397–1408. [Google Scholar] [CrossRef]
- Tallarida, R.J. An overview of drug combination analysis with isobolograms. J. Pharmacol. Exp. Ther. 2006, 319, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies–methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Dhingra, V.K.; Rajpal, S.; Aggarwal, N.; Aggarwaln, J.K.; Shadab, K.; Jain, S.K. Adverse drug reactions observed during DOTS. J. Commun. Dis. 2004, 36, 251–259. [Google Scholar]
- Nageswari, A.D.; Rajanandh, M.G.; Uday, M.K.R.A.; Nasreen, R.J.; Pujitha, R.R.; Prathiksha, G. Effect of rifampin with bio-enhancer in the treatment of newly diagnosed sputum positive pulmonary tuberculosis patients: A double-center study. J. Clin. Tuberc. Mycobac. Dis. 2018, 12, 73–77. [Google Scholar] [CrossRef]
- Wu, D.C.; Chan, W.W.; Metelitsa, A.I.; Fiorillo, L.; Lin, A.N. Pseudomonas skin infection. American J. Clin. Dermatol. 2011, 12, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kalia, N.P.; Suden, P.; Chauhan, P.S.; Kumar, M.; Ram, A.B.; Khan, I.A. Protective efficacy of piperine against Mycobacterium tuberculosis. Tuberculosis 2014, 94, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.J.; Johnson, A.D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Mgbeahuruike, E.E.; Fyhrquist, P.; Julkunen-Tiitto, R.; Vuorela, H.; Holm, Y. Alkaloid-rich crude extracts, fractions and piperamide alkaloids of Piper guineense possess promising antibacterial effects. Antibiotics 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Brown, G.D.; Netea, M.G.; Gow, N.A. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014, 22, 614–622. [Google Scholar] [CrossRef] [Green Version]
- Cockerill, F.R.; Wikler, M.; Bush, K.; Dudley, M.; Eliopoulos, G.; Hardy, D. Performance standards for antimicrobial susceptibility testing: twenty-second informational supplement. Approved Standard—Ninth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]

| Bacteria/ Fungi | Piperine | P. longumine | Tetracycline | Rifampicin | 70% Ethanol | Itraconazole |
|---|---|---|---|---|---|---|
| S. aureus | 15.2 ± 0.31 | 12.4 ± 0.31 | 49.1 ± 0.32 | 47.2 ± 0.17 | NA | NP * |
| P. aeruginosa | 14.4 ± 0.33 | 11.5 ± 0.33 | 34.6 ± 0.33 | 30.3 ± 0.33 | NA | NP * |
| C. albicans | 16.5 ± 0.31 | 18.2 ± 0.31 | NP * | NP * | NA | 15.2 ± 0.33 |
| Ratioof Antibiotics/Compound | S. aureus | P. aeruginosa | C. albicans |
|---|---|---|---|
| Rifam + piperine 1:9 | 7.8 | 15.6 | NP* |
| Rifam + piperine 3:7 | 1.9 | 15.6 | NP * |
| Rifam + piperine 5:5 | 3.9 | 62.5 | NP * |
| Rifam + Piperine 7:3 | 1.9 | 62.5 | NP * |
| Rifam + piperine 9:1 | 7.8 | 15.6 | NP * |
| Rifam + piperlongumine 1:9 | 7.8 | 31.2 | NP * |
| Rifam + piperlongumine 3:7 | 125 | 15.6 | NP * |
| Rifam + piperlongumine 5:5 | 3.9 | 15.6 | NP * |
| Rifam + piperlongumine 7:3 | 62.5 | 15.6 | NP * |
| Rifam + piperlongumine 9:1 | 62.5 | 7.8 | NP * |
| Tetracy + piperine 1:9 | 7.8 | 62.5 | NP * |
| Tetracy + piperine 3:7 | 3.9 | 31.2 | NP * |
| Tetracy + piperine 5:5 | 0.97 | 15.6 | NP * |
| Tetracy + piperine 7:3 | 1.9 | 7.8 | NP * |
| Tetracy + piperine 9:1 | 7.8 | 7.8 | NP * |
| Tetracy + piperlongumine 1:9 | 31.2 | 31.2 | NP * |
| Tetracy + piperlongumine 3:7 | 3.9 | 7.8 | NP * |
| Tetracy + piperlongumine 5:5 | 3.9 | 15.6 | NP * |
| Tetracy + piperlongumine 7:3 | 1.9 | 7.8 | NP * |
| Tetracy + piperlongumine 9:1 | 62.5 | 7.8 | NP * |
| Itracon + piperine 1:9 | NP * | NP * | 3.9 |
| Itracon + piperine 3:7 | NP * | NP * | 3.9 |
| Itracon + piperine 5:5 | NP * | NP * | 7.8 |
| Itracon + piperine 7:3 | NP * | NP * | 7.8 |
| Itracon + piperine 9:1 | NP * | NP * | 15.6 |
| Itracon + piperlongumine 1:9 | NP * | NP * | 31.2 |
| Itracon + piperlongumine 3:7 | NP * | NP * | 7.8 |
| Itracon + piperlongumine 5:5 | NP * | NP * | 7.8 |
| Itracon + piperlongumine 7:3 | NP * | NP * | 3.9 |
| Itracon + piperlongumine 9:1 | NP * | NP * | 31.2 |
| Piperine only | 3.9 | 15.6 | 7.8 |
| Piperlongumine only | 15.6 | 31.2 | 3.9 |
| Rifampicin only | 1.97 | 0.48 | NP * |
| Tetracycline only | 0.97 | 0.97 | NP * |
| Itraconazole only | NP * | NP * | 15.6 |
| Ratio | FIC Index | ||
|---|---|---|---|
| S. aureus | P. aeruginosa | C. albicans | |
| Rifam + piperine 1:9 | 5.9 (AT) | 33.5 (AT) | NP * |
| Rifam + piperine 3:7 | 0.2 (S) | 33.5 (AT) | NP * |
| Rifam + piperine 5:5 | 3.9 (AT) | 8.0 (AT) | NP * |
| Rifam + piperine 7:3 | 1.2 (I) | 5.0 (AT) | NP * |
| Rifam + piperine 9:1 | 12.6 (AT) | 0.4 (S) | NP * |
| Rifam + piperlongumine 1:9 | 4.4 (AT) | 66.0 (AT) | NP * |
| Rifam + piperlongumine 3:7 | 39.0 (AT) | 33.0 (AT) | NP * |
| Rifam + piperlongumine 5:5 | 0,5 (S) | 1.5 (I) | NP * |
| Rifam + piperlongumine 7:3 | 17.0 (AT) | 2.0 (I) | NP * |
| Rifam + piperlongumine 9:1 | 17.0 (AT) | 1.0 (I) | NP * |
| Tetracy + piperine 1:9 | 10.0 (AT) | 68.0 (AT) | NP * |
| Tetracy + piperine 3:7 | 4.5 (AT) | 32.0 (AT) | NP * |
| Tetracy + piperine 5:5 | 0.3 (S) | 0.7 (A) | NP * |
| Tetracy + piperine 7:3 | 2.4 (AT) | 0.7 (A) | NP * |
| Tetracy + piperine 9:1 | 12.1 (AT) | 1.5 (I) | NP * |
| Tetracy + piperlongumine 1:9 | 34.1 (AT) | 33.1 (AT) | NP * |
| Tetracy + piperlongumine 3:7 | 4.1 (AT) | 8.2 (AT) | NP * |
| Tetracy + piperlongumine 5:5 | 1.1 (I) | 2.5 (AT) | NP * |
| Tetracy + piperlongumine 7:3 | 0.9 (A) | 1.5 (I) | NP * |
| Tetracy + piperlongumine 9:1 | 48.9 (AT) | 1.5 (I) | NP * |
| Itracon + piperine 1:9 | NP * | NP * | 0.8 (A) |
| Itracon + piperine 3:7 | NP * | NP * | 1.2 (I) |
| Itracon + piperine 5:5 | NP * | NP * | 4.0 (AT) |
| Itracon + piperine 7:3 | NP * | NP * | 3.0 (AT) |
| Itracon + piperine 9:1 | NP * | NP * | 4.0 (AT) |
| Itracon + piperlongumine 1:9 | NP * | NP * | 10.1 (AT) |
| Itracon + piperlongumine 3:7 | NP * | NP * | 0.7 (A) |
| Itracon + piperlongumine 5:5 | NP * | NP * | 1.3 (I) |
| Itracon + piperlongumine 7:3 | NP * | NP * | 1.0 (I) |
| Itracon + piperlongumine 9:1 | NP * | NP * | 12.0 (AT) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mgbeahuruike, E.E.; Stålnacke, M.; Vuorela, H.; Holm, Y. Antimicrobial and Synergistic Effects of Commercial Piperine and Piperlongumine in Combination with Conventional Antimicrobials. Antibiotics 2019, 8, 55. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8020055
Mgbeahuruike EE, Stålnacke M, Vuorela H, Holm Y. Antimicrobial and Synergistic Effects of Commercial Piperine and Piperlongumine in Combination with Conventional Antimicrobials. Antibiotics. 2019; 8(2):55. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8020055
Chicago/Turabian StyleMgbeahuruike, Eunice Ego, Milla Stålnacke, Heikki Vuorela, and Yvonne Holm. 2019. "Antimicrobial and Synergistic Effects of Commercial Piperine and Piperlongumine in Combination with Conventional Antimicrobials" Antibiotics 8, no. 2: 55. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8020055