Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal pathobionts: An in vitro Microbiological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol
2.2. Plants Extract Preparation
2.3. Microbiological Sample Collection
2.4. Selective Media for Bacterial Growth
2.5. Microbiological Assay
2.5.1. Antimicrobial Susceptibility Assays
2.5.2. Determination of Minimum Inhibitory Concentration (MIC)
2.5.3. Determination of Minimum Bactericidal Concentration (MBC)
2.5.4. Synergistic Antimicrobial Assays
3. Results
3.1. Antibacterial Activity of Antibiotics and Plant Extracts
3.2. MIC and MBC of Plant Extracts
3.3. Synergistic Activity of Plant Extracts with Antimicrobial Agents
3.3.1. The Synergy of C. zeylanicum with Antibiotics
3.3.2. The Synergy of S. presica with Antibiotics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sheiham, A. Oral health, general health, and quality of life. Bull. World Health Organ. 2005, 83, 644–645. [Google Scholar] [PubMed]
- Raitapuro-Murray, T.; Molleson, T.I.; Hughes, F.J. The prevalence of periodontal disease in a Romano-British population c 200-400 AD. Br. Dent. J. 2014, 217, 459–466. [Google Scholar] [CrossRef] [PubMed]
- de Pablo, P.; Chapple, I.L.; Buckley, C.D.; Dietrich, T. Periodontitis in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2009, 5, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.M. Oral health: The silent epidemic. Public. Health. Rep. 2010, 125, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.L.; Leggott, P.; Quinn, R.; Buchanan, S.; Eakle, W.; Chambers, D. Effect of self-administered daily irrigation with 0.02% SnF2 on periodontal disease activity. J. Clin. Periodontol. 1985, 12, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Listgarten, M.A.; Lindhe, J.; Hellden, L. Effect of tetracycline and/or scaling on human periodontal disease. Clinical, microbiological, and histological observations. J. Clin. Periodontol. 1978, 5, 246–271. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Subgingival microflora and periodontal disease. J. Clin. Periodontol. 1979, 6, 351–382. [Google Scholar] [CrossRef] [PubMed]
- Dzink, J.L.; Socransky, S.S.; Haffajee, A.D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J. Clin. Periodontol. 1988, 15, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Wara-aswapati, N.; Pitiphat, W.; Chanchaimongkon, L.; Taweechaisupapong, S.; Boch, J.A.; Ishikawa, I. Red bacterial complex is associated with the severity of chronic periodontitis in a Thai population. Oral. Dis. 2009, 15, 354–359. [Google Scholar] [CrossRef]
- Carranza, F.A.; Newman, M.G.; Takei, H.H.; Klokkevold, P.R. Carranza’s Clinical Periodontology, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Olsvik, B.; Tenover, F.C. Tetracycline resistance in periodontal pathogens. Clin Infect Dis. 1993, 16, S310–S313. [Google Scholar] [CrossRef]
- Pajukanta, R. In vitro antimicrobial susceptibility of Porphyromonas gingivalis to azithromycin, a novel macrolide. Oral Microbiol. Immunol. 1993, 8, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Selection of antimicrobial agents in periodontal therapy. J. Periodontal Res. 2002, 37, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Spellberg, B.; Bartlett, J.G.; Gilbert, D.N. The future of antibiotics and resistance. N. Engl. J. Med. 2013, 368, 299–302. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Periodontology. Treatment of gingivitis and periodontitis Position Paper. J. Periodontol. 1997, 68, 1246–1253. [Google Scholar]
- McCoy, L.C.; Wehler, C.J.; Rich, S.E.; Garcia, R.I.; Miller, D.R.; Jones, J.A. Adverse events associated with chlorhexidine use: Results from the Department of Veterans Affairs Dental Diabetes Study. J. Am. Dent. Assoc. 2008, 139, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Prevention Methods and Programmes for Oral Health. Report of a WHO Expert Committee Technical Report Series 713; World Health Organisation: Geneva, Switzerland, 1987. [Google Scholar]
- Al-Otaibi, M.; Al-Harthy, M.; Söder, B.; Gustafsson, A.; Angmar-Månsson, B. Comparative effect of chewing sticks and toothbrushing on plaque removal and gingival health. Oral Health. Prev. Dent. 2003, 1, 301–307. [Google Scholar]
- Ranasinghe, P.; Pigera, S.; Premakumara, S.G.A.; Priyadarshani Galappaththy, P.; Constantine, R.G.; Katulanda, P. Medicinal properties of ‘true’ cinnamon (Cinnamomum zeylanicum): a systematic review. BMC Complement. Altern. Med. 2013, 13, 275. [Google Scholar] [CrossRef]
- Hunt, D.E.; Jones, J.V.; Dowell, V.R., Jr. Selective medium for the isolation of Bacteroides gingivalis. J. Clin. Microbiol. 1986, 23, 441–445. [Google Scholar]
- Slots, J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J. Clin. Microbiol. 1982, 15, 606–609. [Google Scholar] [PubMed]
- Akemoto, T.; Kurihara, H.; Dahlen, G. Characterization of Bacteroides forsythus isolates. J. Clin. Microbiol. 1997, 35, 1378–1381. [Google Scholar]
- Orth, R.; O’Brien-Simpson, N.; Dashper, S.; Walsh, K.; Reynolds, E. An efficient method for enumerating oral spirochetes using flow cytometry. J. Microbiol. Methods. 2010, 80, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, I.; Albuhadilly, K.A.; Alwindy, S. In vitro assessment of the antibacterial activity of Matricaria chamomile alcoholic extract against pathogenic bacterial strains. Br. Microbiol. Res. J. 2015, 7, 55–61. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Iacobellis, N.S.; de Marco, A.; Capasso, F.; Senatore, F. Antibacterial activity of Coriandrum sativum L. and Foeniculum vulgare Miller Var. vulgare (Miller) essential oils. J. Agric. Food. Chem. 2005, 53, 57–61. [Google Scholar]
- Madduluri, S.; Babu Rao, K.; Sitaram, B. In vitro evaluation of antibacterial activity of five indigenous plants extract against five bacterial pathogens of human. Int. J. Pharm. Pharm. Sci. 2013, 5, 679–684. [Google Scholar]
- Ellen, R.P.; Mc culloch, C.A. Evidence versus empiricism: Rational use of systemic antimicrobial agents for the treatment of periodontitis. Periodontol. 2000. 1996, 10, 29–44. [Google Scholar] [CrossRef]
- Van Winkelhoff, A.J.; Rams, T.E.; Slots, J. Systemic antibiotic therapy in periodontics. Periodontol. 2000. 1996, 10, 45–78. [Google Scholar] [CrossRef]
- Mombelli, A.; Samaranayake, L.P. Topical and systemic antibiotics in the management of periodontal diseases. Int. Dent. J. 2004, 54, 3–14. [Google Scholar] [CrossRef]
- Slots, J. Research, Science and Therapy Committee. Systemic antibiotics in periodontics. J. Periodontol. 2004, 75, 1553–1565. [Google Scholar]
- Haas, A.N.; de Castro, G.D.; Moreno, T.; Susin, C.; Albandar, J.M.; Oppermann, R.V.; Rösing, C.K. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J. Clin. Periodontol. 2008, 35, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, J. In vitro evaluation of anti-caries effect of cinnamon extracts on oral pathogens. Biomed. Res. 2017, 28, 2848–2853. [Google Scholar]
- Julianti, E.; Rajah, K.K.; Fidrianny, I. Antibacterial Activity of Ethanolic Extract of Cinnamon Bark, Honey, and Their Combination Effects against Acne-Causing Bacteria. Sci. Pharm. 2017, 85, 19. [Google Scholar] [CrossRef] [PubMed]
- Ahmada, S.I.; Capoor, M.; Khatoona, F. Phytochemical analysis and growth inhibiting effects of Cinnamomum cassiabark on some pathogenic fungal isolates. J. Chem. Pharm. Res. 2013, 5, 25–32. [Google Scholar]
- Mok, J.S.; Song, K.C.; Choi, N.J.; Yang, H.S. Antibacterial effect of cinnamon (Cinnamomum cassia) bark extract against fish pathogenic bacteria. Korean J. Fish Aqut. Sci. 2001, 34, 545–549. [Google Scholar]
- Bardaji, D.K.; Reis, E.B.; Medeiros, T.C.T.; Lucarini, R.; Crotti, A.E.M.; Martins, C.H.G. Antibacterial activity of commercially available plant-derived essential oils against oral pathogenic bacteria. Nat. Product Res. 2016, 30, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Zainal-Abidin, Z.; Mohd-Said, S.; Abdul-Majid, F.A.; Mustapha, W.A.W.; Jantan, I. Anti-Bacterial Activity of Cinnamon Oil on Oral Pathogens. The Open Conf. Proce. J. 2013, 4, 12–16. [Google Scholar] [CrossRef]
- Aneja, K.R.; Joshi, R.; Sharma, C. Antimicrobial activity of Dalchini (Cinnamomum zeylanicum bark) extracts on some dental caries pathogens. J. Pharm. Res. 2009, 2, 1387–1390. [Google Scholar]
- Al lafi, T.; Ababneh, H. The effect of the extract of the miswak (chewing sticks) used in Jordan and the Middle East on oral bacteria. Int. Dent. J. 1995, 45, 218–222. [Google Scholar]
- Balto, H.; Al-Howiriny, T.; Al-Somaily, A.; Siddiqui, Y.; Al-Sowygh, Z. Screening for the antimicrobial activity of Salvadora persica extracts against Enterococcus faecalis and Candida albicans. Int. J. Phytomed. 2013, 5, 486–492. [Google Scholar]
- Batwa, M.; Bergstrom, J.; Batwa, S.; Al-Otaibi, M. The effectiveness of chewing stick Meswak on plaque removal. Saudi Dent J. 2006, 18, 125–133. [Google Scholar]
- Al-Otaibi, M.; Al-Harthy, M.; Gustafsson, A.; Johansson, A.; Claesson, R.; Angmar-Mansson, B. Subgingival plaque microbiota in Saudi Arabians after use of miswak chewing stick and toothbrush. J. Clin. Periodontol. 2004, 31, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Al-Bagieh, N.; Almas, K. In vitro antibacterial effects of aqueous and alcohol extracts of miswak (chewing sticks). Cairo Dent. J. 1997, 13, 221–224. [Google Scholar]
- Al-Sabawi, N.A.; Al Sheikh, A.K.; Taha, M.Y. The antimicrobial activity of salvadora persica solutions (Miswak-Siwak) as root canal irrigant (A comparative study). J. Pure Appl. Sci. 2007, 4, 69–91. [Google Scholar]
- Sofrata, A.H.; Claesson, R.L.K.; Lingstrom, P.K.; Gustafsson, A.K. Strong antibacterial effect of miswak against oral microorganisms associated with periodontitis and caries. J. Periodontol. 2008, 79, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Alireza, R.G.A.; Afsaneh, R.; Hosein, M.S.S.; Siamak, Y.; Afshin, K.; Zeinab, K.; Mahvash, M.J.; Reza, R.A. Inhibitory activity of Salvadora persica extracts against oral bacterial strains associated with periodontitis: An in vitro study. J. Oral. Biol. Craniofac. Res. 2014, 4, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, Z.; Narasimhan, S.; Haridoss, M.; Vennila, R.; Vaidyanathan, R. Punica granatum rind extract: Antibiotic potentiator and efflux pump inhibitor of multidrug-resistant Klebsiella pneumoniae clinical isolates. Asian. J. Pharm. Clin. Res. 2017, 10, 1–5. [Google Scholar] [CrossRef]
- Cristo, J.S.; Matias, E.F.F.; Figueredo, F.G.; Santos, J.F.S.; Pereira, N.L.F.; Junior, J.G.A.S.; Aquinoa, P.E.A.; Nogueira, M.N.F.; Ribeiro-Filhoa, J.; Cunha, F.A.B.; et al. HPLC profile and antibiotic-modifying activity of Azadirachta indica A. Juss (Meliaceae). Ind. Crops. Prod. 2016, 94, 903–908. [Google Scholar] [CrossRef]
- Cha, S.M.; Han, S.B.; Lee, Y.S.; Cha, J.D. Synergistic Effect of the Ethanol Extract of Alismatis rhizoma against Oral Pathogens. J. Oral. Bio. 2015, 2, 7. [Google Scholar]
- Al-Hebshi, N.; Al-Haroni, M.; Skaug, N. In vitro antimicrobial and resistance-modifying activities of aqueous crude khat extracts against oral microorganisms. Arch. Oral. Biol. 2006, 51, 183–188. [Google Scholar] [CrossRef]


| Bacteria | Media |
|---|---|
| P. gingivalis | (Columbia agar base *, Bacitracin *, Colistin *, Nalidixic acid *) [21] |
| A. actinomycetemcomitans | (Trypticase soy *, Bacitracin *, Vancomycin ** (TSBV)) [22] |
| T. forsythia | (Tryptic soy broth *, Yeast extract **, Vit. K *, N-Acetylmuramic acid *) [23] |
| T. denticola | (Oral bacteria growth medium (OBGM)) [24] |
| Bacteria | Ethanolic Extract | |||||
|---|---|---|---|---|---|---|
| C. zeylanicum | S. presica | |||||
| Zone (mm) (mean ± SD) | MIC (mg/mL) (mean ± SD) | MBC (mg/mL) (mean ± SD) | Zone (mm) (mean ± SD) | MIC (mg/mL) (mean ± SD) | MBC (mg/mL) (mean ± SD) | |
| P. gingivalis | 18 ± 1.5 | 3.12 ± 0.65 | 12.5 ± 1.35 | 15 ± 0.35 | 6.25 ± 1.24 | 50 ± 6.21 |
| T. denticola | 13 ± 1.0 | 6.25 ± 1.24 | 50 ± 5.24 | 14 ± 1.75 | 6.25 ± 1.15 | 25 ± 3.25 |
| T. forsythia | 21 ± 1.75 | 1.56 ± 0.24 | 6.25 ± 0.68 | 19 ± 1.56 | 3.12 ± 0.85 | 11.5 ± 3.21 |
| A. actinomycetemcomitans | 8 ± 0.75 | 12.5 ± 3.25 | 75 ± 8.23 | 10 ± 2.0 | 12.5 ± 2.25 | 50 ± 6.35 |
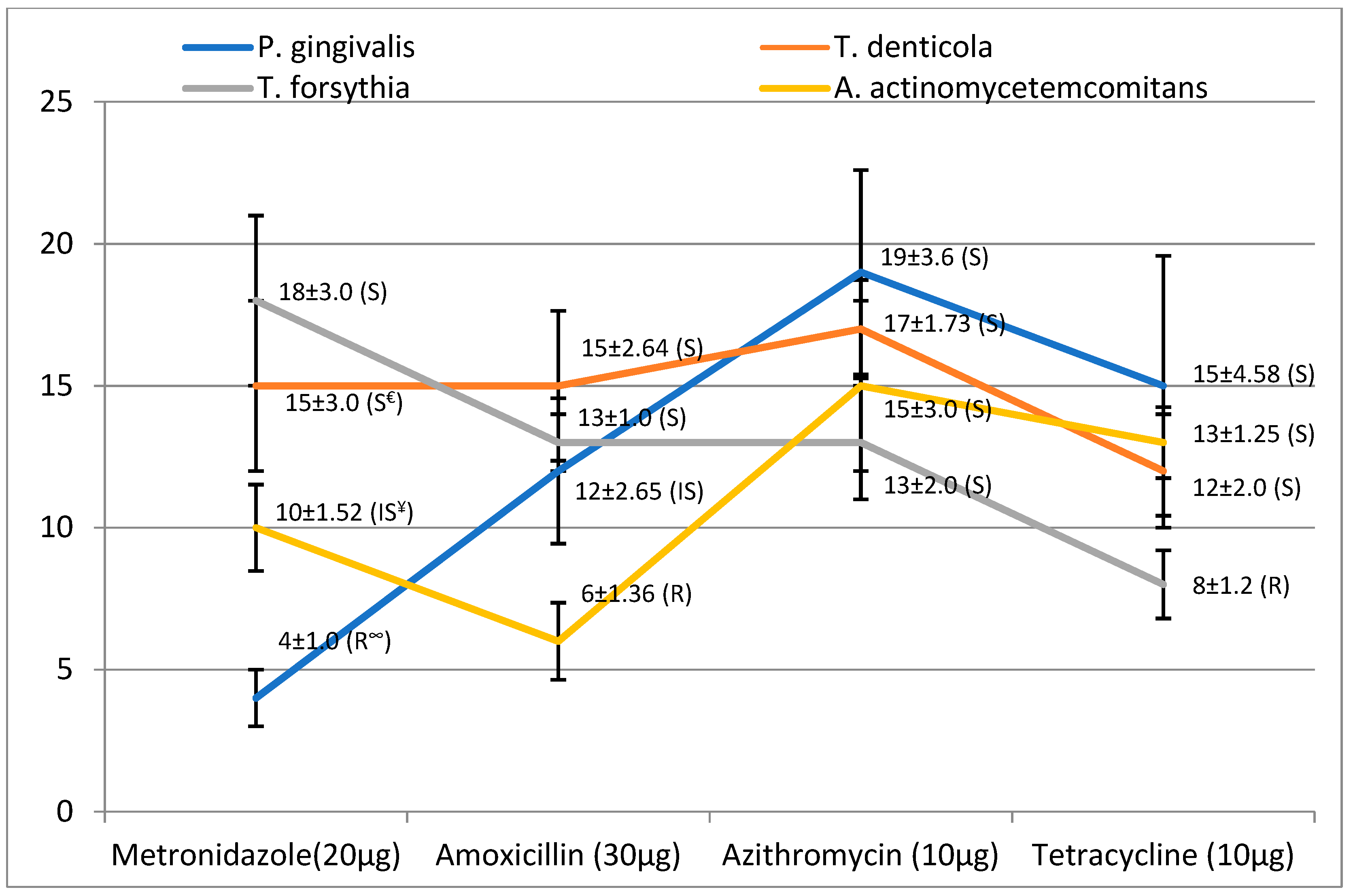
| Bacteria | Antibiotics (anti.) | MIZ * with anti. (mm) (mean ± SD) | MIZ with C. zeylanicum (mm) (mean ± SD) | MIZ with anti. and C. zeylanicum (mm) (mean ± SD) | Outcome |
|---|---|---|---|---|---|
| P. gingivalis | Metronidazole | 4 ± 1.0 | 18 ± 1.5 | 20 ± 3.25 | Antagonism |
| Amoxicillin | 12 ± 2.65 | 30 ± 4.12 | Additive | ||
| Azithromycin | 19 ± 3.6 | 40 ± 4.85 | Synergism | ||
| Tetracycline | 15 ± 4.58 | 36 ± 4.02 | Synergism | ||
| T. denticola | Metronidazole | 15 ± 3.0 | 13 ± 1.0 | 27 ± 3.21 | Antagonism |
| Amoxicillin | 15 ± 2.64 | 30 ± 3.65 | Synergism | ||
| Azithromycin | 17 ± 1.73 | 34 ± 3.75 | Synergism | ||
| Tetracycline | 12 ± 2.0 | 21 ± 2.85 | Antagonism | ||
| T. forsythia | Metronidazole | 18 ± 3.0 | 21 ± 1.75 | 34 ± 4.01 | Antagonism |
| Amoxicillin | 13 ± 1.0 | 38 ± 4.12 | Synergism | ||
| Azithromycin | 13 ± 2.0 | 30 ± 3.12 | Antagonism | ||
| Tetracycline | 8 ± 1.2 | 29 ± 3.04 | Additive | ||
| A. actinomycetemcomitans | Metronidazole | 10 ± 1.52 | 8 ± 1.75 | 20 ± 2.26 | Synergism |
| Amoxicillin | 6 ± 1.36 | 14 ± 1.96 | Additive | ||
| Azithromycin | 15 ± 3.0 | 21 ± 3.05 | Antagonism | ||
| Tetracycline | 13 ± 1.25 | 18 ± 2.85 | Antagonism |
| Bacteria | Antibiotics (anti.) | MIZ * with anti. (mm) (mean ± SD) | MIZ with S. presica (mm) (mean ± SD) | MIZ with anti. and S. presica (mm) (mean ± SD) | Outcome |
|---|---|---|---|---|---|
| P. gingivalis | Metronidazole | 4 ± 1.0 | 15 ± 0.35 | 18 ± 1.68 | Antagonism |
| Amoxicillin Azithromycin Tetracycline | 12 ± 2.65 19 ± 3.6 15 ± 4.58 | 31 ± 2.15 34 ± 3.5 33 ± 3.78 | Synergism Additive Synergism | ||
| T. denticola | Metronidazole | 15 ± 3.0 | 14 ± 1.75 | 29 ± 2.69 | Additive |
| Amoxicillin | 15 ± 2.64 | 27 ± 1.45 | Antagonism | ||
| Azithromycin | 17 ± 1.73 | 31 ± 2.65 | Additive | ||
| Tetracycline | 12 ± 2.0 | 28 ± 2.96 | Synergism | ||
| T. forsythia | Metronidazole | 18 ± 3.0 | 19 ± 1.56 | 32 ± 3.02 | Antagonism |
| Amoxicillin | 13 ± 1.0 | 37 ± 3.47 | Synergism | ||
| Azithromycin | 13 ± 2.0 | 32 ± 4.02 | Additive | ||
| Tetracycline | 8 ± 1.2 | 31 ± 2.58 | Synergism | ||
| A. actinomycetemcomitans | Metronidazole | 10 ± 1.52 | 10 ± 2.0 | 27 ± 1.78 | Synergism |
| Amoxicillin | 6 ± 1.36 | 14 ± 0.85 | Antagonism | ||
| Azithromycin | 15 ± 3.0 | 28 ± 3.26 | Synergism | ||
| Tetracycline | 13 ± 1.25 | 22 ± 1.52 | Antagonism |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saquib, S.A.; AlQahtani, N.A.; Ahmad, I.; Kader, M.A.; Al Shahrani, S.S.; Asiri, E.A. Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal pathobionts: An in vitro Microbiological Study. Antibiotics 2019, 8, 89. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8030089
Saquib SA, AlQahtani NA, Ahmad I, Kader MA, Al Shahrani SS, Asiri EA. Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal pathobionts: An in vitro Microbiological Study. Antibiotics. 2019; 8(3):89. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8030089
Chicago/Turabian StyleSaquib, Shahabe Abullais, Nabeeh Abdullah AlQahtani, Irfan Ahmad, Mohammed Abdul Kader, Sami Saeed Al Shahrani, and Elyas Ali Asiri. 2019. "Evaluation and Comparison of Antibacterial Efficacy of Herbal Extracts in Combination with Antibiotics on Periodontal pathobionts: An in vitro Microbiological Study" Antibiotics 8, no. 3: 89. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8030089





