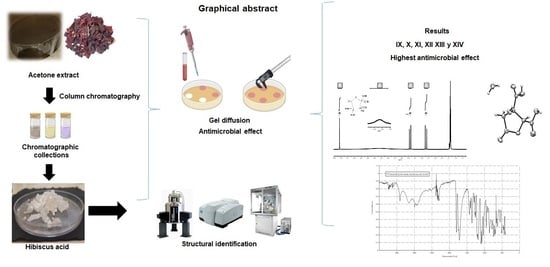
Hibiscus Acid and Chromatographic Fractions from Hibiscus Sabdariffa Calyces: Antimicrobial Activity against Multidrug-Resistant Pathogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hibiscus Sabdariffa Extract
2.2. Chromatographic Fractionation of Acetone Extract
2.3. Extraction of Hibiscus Acid
2.4. Structural Identification of Hibiscus Acid
2.4.1. Nuclear Magnetic Resonance Spectroscopy
2.4.2. Infrared Spectroscopy with Attenuated Total Reflection
2.4.3. X-ray Crystallography
2.4.4. Differential Scanning Calorimetry
2.5. Determination of the Anti-microbial Effect of Acetone Extract, Chromatographic Collections and Hibiscus Acid
2.5.1. Preparation of Test Solutions
2.5.2. Bacterial Strains
2.5.3. Preparation of Bacterial Strains
2.5.4. Anti-microbial Activity of Acetone Extract, Chromatographic Collections and Hibiscus Acid
2.6. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration
2.7. Measurement of Permeability with Crystal Violet
2.8. Statistical Analysis
3. Results and Discussion
3.1. Anti-microbial Activity of Acetonic Extract of Hibiscus Sabdariffa
3.2. Anti-microbial Activity of Chromatographic Collections against Pathogenic Bacteria
3.3. Obtaining Presumptive Crystals of Hibiscus Acid from the Acetonic Extract
3.4. Structural Identification of Hibiscus Acid
3.4.1. 1H NMR Spectrum
3.4.2. Infrared Spectroscopy
3.4.3. X-ray Crystallography
3.4.4. Hibiscus Acid Melting Point by Differential Scanning Calorimetry
3.5. Anti-microbial Effect of Hibiscus Acid
3.6. Determination of the MIC and MBC of the Acetone Extract and Hibiscus Acid
3.7. Measurement of Permeability with Crystal Violet
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gómez-Aldapa, A.C.A.; Torres-Vitela, M.D.; Villarruel-López, A.; Castro-Rosas, J. The role of foods in Salmonella infections. In Salmonella. A Dangerous Foodborne Pathogen; Mahmoud, B.S.M., Ed.; Intech: Rijeka, Croatia, 2012; Volume 2, pp. 21–46. [Google Scholar]
- Lamas, J.A.; Miranda, J.M.; Regal, P.; Vazquez, B.; Franco, C.M.; Cepeda, A. A comprehensive review of non-enterica subspecies of Salmonella enterica. Microbiol. Res. 2008, 206, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Cravioto, A.; Tello, A.; Navarro, A.; Ruiz, J.; Villafán, H.; Uribe, F.; Eslava, C. Association of Escherichia coli HEp-2 adherence patterns with type and duration of diarrhoea. Lancet 1991, 337, 262–264. [Google Scholar] [CrossRef]
- Estrada-García, T.; Cerna, J.F.; Thompson, M.R.; López-Saucedo, C. Faecal contamination and enterotoxigenic Escherichia coli in street-vended chili sauces in Mexico and its public health relevance. Epidemiol. Infect. 2002, 129, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Estrada-García, T.; López- Saucedo, C.; Thompson, B.R.; Abonce, M.; Lopez-Hernández, D.; Santos, J.I.; Rosado, J.L.; Dupont, H.L.; Long, K.Z. Association of diarrheagenic Escherichia coli pathotypes with infection and diarrhea among Mexican children and association of atypical enteropathogenic E. coli with acute diarrhea. J. Clin. Microbiol. 2009, 47, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, G.L.; Monroy, E.; García-González, O.; Alonso, J.; Negrete, E.; Vaca, S. Two or more enteropathogens are associated with diarrhoea in Mexican children. Ann. Clin. Microbiol. Antimicrob. 2006, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.A.; Mathewson, J.J.; Jiang, Z.D.; Ericsson, C.D.; DuPont, H.L. Enteric pathogens in Mexican sauces of popular restaurants in Guadalajara, Mexico, and Houston, Texas. Ann. Intern. Med. 2002, 136, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Cerna-Cortés, J.F.; Vega-Negrete, W.; Ortega-Villegas, M.A.; Zaidi, M.B.; Estrada-García, T. Consumption of street-vended beverage a potential exposure risk for non-O157 enterohemorrhagic Escherichia coli infection: The importance of testing for virulence loci. Clin. Infect. Dis. 2012, 54, 154–155. [Google Scholar] [CrossRef] [PubMed]
- López-Saucedo, C.; Cerna, C.J.F.; Estrada-García, T. Non-O157 Shiga toxin-producing Escherichia coli is the most prevalent diarrheagenic E. coli pathotype in street-vended taco dressings in Mexico City. Clin. Infect. Dis. 2010, 50, 450–451. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Paredes, M.; Okhuysen, P.C.; Flores, J.; Mohamed, J.A.; Padda, R.S.; González-Estrada, A.; Haley, C.A.; Carlin, L.G.; Nair, P.; DuPont, H.L. Seasonality of diarrheagenic Escherichia coli pathotypes in the US students acquiring diarrhea in Mexico. J. Travel Med. 2011, 18, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 53, 28–52. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Foodborne antimicrobial resistance as a biological hazard. Scientific opinion of the panel on biological hazards. EFSA J. 2008, 765, 1–87. [Google Scholar] [CrossRef]
- El Kamali, H.H.; Mohammed, M.F. Antibacterial activity of Hibiscus sabdariffa, Acacia seyal var. seyal and Sphaeranthus suaveolens var. suaveolens against upper respiratory tract pathogens. Sudan J. Med. Sci. 2006, 1, 121–126. [Google Scholar] [CrossRef]
- Fullerton, M.; Khatiwada, J.; Johnson, J.U.; Davis, S.; Williams, L.L. Determination of antimicrobial activity of sorrel (Hibiscus sabdariffa) on Escherichia coli O157:H7 isolated from food, veterinary, and clinical samples. J. Med. Food 2011, 14, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aldapa, C.A.; Cerna-Cortés, J.F.; Rangel-Vargas, E.; Torres-Vitela, M.D.; Villarruel López, A.; Gutiérrez-Alcántara, E.J.; Castro-Rosas, J. Presence of multidrug-resistant Shiga toxin-producing Escherichia coli, enteropathogenic E. coli and enterotoxigenic E. coli, on raw nopalitos (Opuntia ficus-indica L.) and in nopalitos salads from local retail markets in Mexico. Foodborne Pathog. Dis. 2016, 13, 269–274. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Portillo-Torres, L.A.; Villagómez-Ibarra, J.R.; Rangel-Vargas, E.; Téllez-Jurado, A.; Cruz-Gálvez, A.M.; Castro-Rosas, J. Survival of foodborne bacteria on strawberries and antibacterial activities of Hibiscus sabdariffa extracts and chemical sanitizers on strawberries. J. Food Saf. 2018, 38, 1–8. [Google Scholar] [CrossRef]
- Gutiérrez-Alcántara, E.J.; Gómez-Aldapa, C.A.; Román-Gutiérrez, A.D.; Rangel-Vargas, E.; González-Olivares, L.G.; Castro-Rosas, J. Antimicrobial activity of roselle Hibiscus sabdariffa calyx extracts on culture media and carrots against multidrug-resistant Salmonella strains isolated from raw carrots. J. Food Saf. 2016, 36, 450–458. [Google Scholar] [CrossRef]
- Gutiérrez-Alcántara, E.J.; Rangel-Vargas, E.; Gómez-Aldapa, C.A.; Falfán-Cortés, R.N.; Rodríguez-Marín, M.L.; Godínez-Oviedo, A.; Cortés-López, H.; Castro-Rosas, J. Antibacterial effect of roselle extracts (Hibiscus sabdariffa), sodium hypochlorite and acetic acid against multidrug-resistant Salmonella strains isolated from tomatoes. Lett. Appl. Microbiol. 2016, 62, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.S.; Seok, J.H.; Kim, Y.H.; Eun, J.S.; Oh, S.H. Antimicrobial and antioxidative effects of roselle (Hibiscus sabdariffa L.) flower extract and its fractions on skin microorganisms and oxidation. Food Sci. Biotechnol. 2007, 16, 409–414. [Google Scholar]
- Liu, K.S.; Tsao, S.M.; Yin, M.C. In vitro antibacterial activity of roselle calyx and protocatechuic acid. Phytother. Res. 2005, 19, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cabrera, M.; Hernández-Morales, J.; Leyva-Ruelas, G.; Salinas-Moreno, Y.; Soto-Rojas, L.; Castro-Rosas, J. Influence of variety and extraction solvent on antibacterial activity of roselle (Hibiscus sabdariffa L.) calyxes. J. Med. Plant Res. 2013, 7, 2319–2322. [Google Scholar] [CrossRef]
- Olaleye, M.T. Cytotoxicity and antibacterial activity of methanolic extract of Hibiscus sabdariffa. J. Med. Plant Res. 2007, 1, 9–13. [Google Scholar]
- Rangel-Vargas, E.; Gutiérrez-Alcántara, E.J.; Gómez-Aldapa, C.A.; Falfán-Cortés, R.N.; Segovia-Cruz, J.A.; Salas-Rangel, L.P.; Castro-Rosas, J. Antibacterial activity of roselle calyx extracts, sodium hypochlorite, colloidal silver and acetic acid against multidrug-resistant Salmonella serotypes isolated from coriander. J. Food Saf. 2017, 37, 1–10. [Google Scholar] [CrossRef]
- Mahadevan, N.; Kamboj, P. Hibiscus sabdariffa Linn–An overview. Nat. Prod. Radiance 2009, 8, 77–83. [Google Scholar]
- Chao, C.Y.; Yin, M.C. Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 2009, 6, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Chao, C.Y. Anti-Campylobacter, anti-aerobic, and anti-oxidative effects of roselle calyx extract and protocatechuic acid in ground beef. Int. J. Food Microbiol. 2008, 127, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Debon, R.; Alonso-Villaverde, C.; Aragones, G.; Rodriguez-Medina, I.; Rull, A.; Micol, V. The aqueous extract of Hibiscus sabdariffa calices modulates the production of monocyte chemoattractant protein-1 in humans. Phytomedicine 2010, 17, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hansawasdi, C.; Kawabata, J.; Kasai, T. Hibiscus Acid as an Inhibitor of Starch Digestion in the Caco-2 Cell Model System. Biosci. Biotechnol. Biochem. 2001, 64, 1041–1043. [Google Scholar] [CrossRef] [PubMed]
- Zheoat, A.M.; Gray, A.I.; Ogbaji, J.I.; Ferro, A.B.; Drummond, R.B. Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia 2019, 134, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gálvez, A.M.; Gómez-Aldapa, C.A.; Villagómez-Ibarra, J.R.; Chavarría-Hernández, N.; Rodríguez-Baños, J.; Rangel-Vargas, E.; Castro-Rosas, J. Antibacterial effect against foodborne bacteria of plants used in traditional medicine in central Mexico: Studies in vitro and in raw beef. Food Control. 2013, 32, 289–295. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aldapa, C.A.; Segovia-Cruz, J.A.; Cerna-Cortés, J.F.; Rangel-Vargas, E.; Salas-Rangel, L.P.; Gutiérrez-Alcántara, E.J.; Castro-Rosas, J. Prevalence and behavior of multidrug-resistant Shiga toxin-producing Escherichia coli, enteropathogenic E. coli and enterotoxigenic E. coli on coriander. Food Microbiol. 2016, 59, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility tessting; twenty-fifth informational supplement. CLSI 2005, 35, 1–249. [Google Scholar]
- Vanden, B.D.A.; Vlietinck, A.J. Screening methods for antibacterial and antiviral agents from higher plants. In Methods in Plant Biochemistry; Dey, P.M., Harborne, J.B., Eds.; Academic Press: London, UK, 1991; Volume 2, pp. 47–69. [Google Scholar]
- Devil, K.P.; Nisha, S.A.; Sakthivel, R.; Karutha, S.P. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Sosa, R.; Gastélum-Franco, M.G.; Camacho-Davila, A.; Torres-Muñoz, J.; Nevárez-Morrillón, G.V. Extracts of Mexican oregano (Lippia berlandieri Schauer) with antioxidant and antimicrobial activity. Food Bioprocess Technol. 2010, 3, 434–440. [Google Scholar] [CrossRef]
- Kuete, V.; Kamga, J.; Sandjo, L.P.; Ngameni, B.; Poumale, H.M.P.; Ambassa, P.; Ngadjui, B.T. Antimicrobial activities of the methanol extract, fractions and compounds from Ficus polita Vahl. (Moraceae). BMC Complement. Altern. Med. 2011, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, T.H.A.; Le, H.H.; Kitsamone, S.; Nguyen, T.U.; Nguyen, Q.H. In vitro antibacterial activity of quercetin containing extract from Hibiscus sabdariffa L. calyces. VNU J. Sci. Nat. Sci. Technol. 2016, 32, 147–152. [Google Scholar]
- Ibnusaud, I.; Thomas, P.T.; Rani, R.N.; Sasi, P.V.; Beena, T.; Hisham, A. Chiral γ-butyrolactones related to optically active 2-hydroxycitric acids. Tetrahedron 2002, 58, 4887–4892. [Google Scholar] [CrossRef]
- He, K.; Roller, M. The use of nuclear magnetic resonance spectroscopy for the identification of biomarkers for quality control of plant extracts. In Formulating, Packaging, and Marketing of Natural Cosmetic Products; Dayan, N., Kromidas, L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Volume 19, pp. 361–385. [Google Scholar]
- Zheoat, A.M.; Gray, A.I.; Igoli, J.O.; Kennedy, A.R.; Ferro, V.A. Crystal structures of hibiscus acid and hibiscus acid dimethyl ester isolated from Hibiscus sabdariffa (Malvaceae). Acta Crystallogr. 2017, 73, 1368–1371. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, D.M.; Porzel, A.; Frolov, A.; El Seedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.Y.; Zeng, L.; Liu, J.H.; Chen, S. Characterization of Salmonella food isolates with concurrent resistance to ceftriaxone and ciprofloxacin. Foodborne Pathog. Dis. 2013, 10, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and Its pharmacological potential. ISRN Pharmacol. 2014, 95, 29–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djeussi, D.E.; Noumedem, J.A.; Seukep, J.A.; Fankam, A.G.; Voukeng, I.K.; Tankeo, S.B.; Nkuete, A.H.; Kuete, V. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. BMC Complement. Altern. Med. 2013, 13, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdallah, E.M. Antibacterial activity of Hibiscus sabdariffa L. calyces against hospital isolates of multidrug resistant Acinetobacter baumannii. J. Acute Dis. 2016, 5, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Tsuchido, T.; Katsui, N.; Takeuchi, A.; Takano, M.; Shibasaki, I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl. Environ. Microbiol. 1985, 50, 298–303. [Google Scholar] [PubMed]
- Haque, H.; Russel, A.D. Effect of ethylenediaminetetraacetic acid and related chelating agents on whole cells of gram-negative bacteria. Antimicrob. Agents Chemother. 1974, 5, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Fraction Number | Solvent Ratio Used in Chromatography Column as Mobile Phase |
|---|---|
| 1–37 | Hexane |
| 38–59 | 90–10% Hexane–ethyl acetate |
| 60–131 | 80–20% Hexane–ethyl acetate |
| 132–277 | 70–30% Hexane–ethyl acetate |
| 278–348 | 60–40% Hexane–ethyl acetate |
| 349–396 | 50–50% Hexane–ethyl acetate |
| 397–441 | 40–60% Hexane–ethyl acetate |
| 442–486 | 30–70% Hexane–ethyl acetate |
| 487–535 | 20–80% Hexane–ethyl acetate |
| 536–572 | 10–90% Hexane–ethyl acetate |
| 573–616 | Ethyl acetate |
| 617–660 | 90–10% Ethyl acetate–methanol |
| 661–693 | 80–20% Ethyl acetate–methanol |
| 694–731 | 70–30% Ethyl acetate–methanol |
| 732–771 | 60–40% Ethyl acetate–-methanol |
| 772–794 | 50–50% Ethyl acetate–methanol |
| 795–810 | 40–60% Ethyl acetate–methanol |
| 811–842 | 30–70% Ethyl acetate–methanol |
| 843–868 | 20–80% ethyl acetate–methanol |
| 869–886 | 10–90% ethyl acetate–methanol |
| 887–903 | Methanol |
| Collection | Fraction | Collection | Fraction |
|---|---|---|---|
| I | 1–42 | XIV | 285–379 |
| II | 43–46 | XV | 380–407 |
| III | 47–59 | XVI | 408–447 |
| IV | 60–62 | XVII | 448–473 |
| V | 63–68 | XVIII | 474–564 |
| VI | 69–107 | XIX | 565–584 |
| VII | 108–116 | XX | 585–620 |
| VIII | 117–132 | XXI | 621–695 |
| IX | 133–155 | XXII | 696–740 |
| X | 156–176 | XXIII | 741–792 |
| XI | 180–200 | XXIV | 793–867 |
| XII | 201–256 | XXV | 868–903 |
| XIII | 257–284 |
| Collection | Salmonella C1 | Salmonella C65 | Salmonella C63 | EHEC A | EIEC MAC B | E. coli C558 | E. coli C636 | EPEC MAC A |
|---|---|---|---|---|---|---|---|---|
| VI 1 | 7.0 ± 0.2 b, 2 | 7.2 ± 0.4 a | - a | - a | - a | - a | - a | - a |
| VII | - a | 7.5 ± 0.1 ab | - a | - a | - a | - a | - a | - a |
| IX | 10.8 ± 0.2 g | 12.6 ± 0.2 gh | 13.3 ± 0.2 i | 11.9 ± 0.2 gh | 12.2 ± 0.3 h | 9.3 ± 0.2 bcd | 10.1 ± 0.5 efg | 12.0 ± 0.4 h |
| X | 13.5 ± 0.4 h | 11.5 ± 0.4 f | 13.3 ± 0.2 i | 12.3 ± 0.2 h | 11.6 ± 0.6 fgh | 11.5 ± 0.2 g | 11.6 ± 0.7 h | 12.4 ± 0.2 h |
| XI | 13.5 ± 0.2 h | 11.6 ± 0.2 fg | 15.2 ± 0.1 j | 14.2 ± 0.2 i | 12.5 ± 0.4 h | 11.5 ± 0.3 g | 13.2 ± 0.3 i | 11.5 ± 0.2 gh |
| XII | 11.1 ± 0.6 g | 12.6 ± 0.2 gh | 10.1 ± 0.1 defgh | 9.6 ± 0.5 ef | 10.5 ± 0.6 de | 11.8 ± 0.3 g | 10.2 ± 0.2 efg | 9.5 ± 0.5 cde |
| XIII | 10.5 ± 0.3 fg | 15.2 ± 0.5 i | 11.0 ± 0.1 h | 11.5 ± 0.3 g | 11.8 ± 0.5 gh | 11.4 ± 0.3 fg | 10.9 ± 0.1 gh | 11.0 ± 0.3 fg |
| XIV | 10.9 ± 0.4 g | 11.9 ± 0.5 fg | 10.7 ± 0.6 gh | 10.2 ± 0.3 f | 10.5 ± 0.1 def | 11.5 ± 0.4 g | 9.8 ± 0.4 def | 10.3 ± 0.5 def |
| XV | 10.8 ± 0.4 g | 13.5 ± 0.2 h | 10.5 ± 0.3 fgh | 9.5 ± 0.3 ef | 11.1 ± 0.5 efg | 9.6 ± 0.5 cde | 10.6 ± 0.4 fg | 9.6 ± 0.1 cde |
| XVI | 9.5 ± 0.1 def | 9.7 ± 0.4 e | 9.8 ± 0.4 defg | 9.9 ± 0.1 f | 10.1 ± 0.1 bcde | 10.4 ± 0.3 ef | 9.6 ± 0.4 de | 10.4 ± 0.1 ef |
| XVII | 9.1 ± 0.2 cde | 9.7 ± 0.3 e | 9.5 ± 0.4 de | 10.0 ± 0.4 f | 10.3 ± 0.7 cde | 9.7 ± 0.1 cde | 9.8 ± 0.4 def | 9.4 ± 0.7 cd |
| XVIII | 9.4 ± 0.6 de | 9.6 ± 0.3 e | 9.6 ± 0.5 def | 9.1 ± 0.2 de | 9.9 ± 0.6 bcd | 10.1 ± 0.7 de | 9.5 ± 0.1 de | 8.8 ± 0.2 c |
| XIX | 8.7 ± 0.2 cde | 8.5 ± 0.7 bcd | 9.3 ± 0.3 cd | 8.4 ± 0.3 cd | 9.5 ± 0.4 bcd | 9.3 ± 0.2 bcd | 7.4 ± 0.5 b | 7.8 ± 0.1 b |
| XX | 8.9 ± 0.4 cde | 9.5 ± 0.0 de | 9.9 ± 0.1 defg | 9.0 ± 0.2 de | 9.3 ± 0.1 bc | 9.2 ± 0.3 bcd | 8.2 ± 0.1 bc | 8.7 ± 0.1 bc |
| XXI | 8.1 ± 0.1 c | 8.8 ± 0.3 cde | 8.3 ± 0.2 b | 7.8 ± 0.2 bc | 9.0 ± 0.1 b | 8.3 ± 0.3 b | 8.5 ± 0.4 c | 8.9 ± 0.4 c |
| XXII | 8.5 ± 0.4 cd | 8.3 ± 0.2 bc | 8.6 ± 0.2 bc | 7.5 ± 0.2 b | - a | - a | - a | 10.0 ± 0.2 de |
| XXIII | 9.6 ± 0.5 ef | 9.1 ± 0.4 cde | 10.3 ± 0.2 efgh | 8.6 ± 0.1 d | 9.0 ± 0.7 b | 8.9 ± 0.5 bc | 9.1 ± 0.1 cd | 10.1 ± 0.3 def |
| Experimental Data | |
|---|---|
| Empirical Formula | C6H6O7 • H2O |
| Molecular weight | 208.12 |
| Temperature (K) | 293(2) |
| Crystal system, space group | orthorhombic, P212121 |
| Unit cell dimensions (Å, °) | |
| a | 8.2069(2) |
| b | 9.9228(2) |
| c | 10.1747(2) |
| α(°) | 90 |
| β(°) | 90 |
| γ(°) | 90 |
| Volume (Å3) | 828.58(3) |
| Z | 5 |
| Radiation type | CuKα (λ = 1.54184 Å) |
| μ (mm−1) | 1.797 |
| ρcalc (g cm−3) | 2.096 |
| F (000) | 545.00 |
| 2θ range for data collection | 12.46–155.038 |
| Index Ranges | −10 ≤ h ≤ 10, −11 ≤ k ≤ 12, −11 ≤ l ≤ 12 |
| Absorption Correction | Multi-scan |
| Collected Reflections | 11147 |
| Independent Reflections | 1754 (Rint = 0.0293) |
| Data/Restraints/Parameters | 1754/0/133 |
| Goodness-of-fit on F2 | 1.077 |
| R1, wR2 [I ≥ σ2s(I)] | 0.0309, 0.0859 |
| R1, wR2 [all data] | 0.0314, 0.0864 |
| Largest Difference Peak/Hole (e Å−3) | 0.25 and −0.21 |
| Flack and Hooft Parameters | 0.05(6) and 0.07(5) |
| Inverted Flack and Hooft Parameters | 0.95(6) and 0.93(5) |
| Bacteria | Treatment | |
|---|---|---|
| Acetone extract | Hibiscus Acid | |
| Salmonella C1 1 | 12.6 ± 0.1 a | 16.0 ± 0.4 b |
| Salmonella C65 | 10.8 ± 0.3 a | 14.5 ± 0.1 b |
| Salmonella C63 | 10.3 ± 0.3 a | 11.6 ± 0.2 b |
| EHEC A | 10.7 ± 0.4 a | 10.0 ± 0.3 a |
| EIEC MAC B | 11.5 ± 0.1 a | 13.4 ± 0.6 b |
| E. coli C558 | 11.8 ± 0.1 a | 11.6 ± 0.4 a |
| E. coli C636 | 10.4 ± 0.5 a | 11.1 ± 0.2 a |
| EPEC MAC A | 9.8 ± 0.1 a | 10.5 ± 0.3 b |
| Bacteria | Acetone Extract | Hibiscus Acid | ||||
|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC/MBC | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | |
| Salmonella C1 | 7 | 10 | 1.4 | 4 | 5 | 1.3 |
| Salmonella C65 | 7 | 7 | 1.0 | 7 | 7 | 1.0 |
| Salmonella C63 | 7 | 10 | 1.4 | 5 | 7 | 1.4 |
| EHEC A | 7 | 10 | 1.4 | 5 | 7 | 1.4 |
| EIEC MAC B | 7 | 10 | 1.4 | 5 | 7 | 1.4 |
| E. coli C558 | 7 | 10 | 1.4 | 5 | 7 | 1.4 |
| E. coli C636 | 7 | 10 | 1.4 | 5 | 5 | 1.0 |
| EPEC MAC A | 7 | 10 | 1.4 | 4 | 7 | 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portillo-Torres, L.A.; Bernardino-Nicanor, A.; Gómez-Aldapa, C.A.; González-Montiel, S.; Rangel-Vargas, E.; Villagómez-Ibarra, J.R.; González-Cruz, L.; Cortés-López, H.; Castro-Rosas, J. Hibiscus Acid and Chromatographic Fractions from Hibiscus Sabdariffa Calyces: Antimicrobial Activity against Multidrug-Resistant Pathogenic Bacteria. Antibiotics 2019, 8, 218. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040218
Portillo-Torres LA, Bernardino-Nicanor A, Gómez-Aldapa CA, González-Montiel S, Rangel-Vargas E, Villagómez-Ibarra JR, González-Cruz L, Cortés-López H, Castro-Rosas J. Hibiscus Acid and Chromatographic Fractions from Hibiscus Sabdariffa Calyces: Antimicrobial Activity against Multidrug-Resistant Pathogenic Bacteria. Antibiotics. 2019; 8(4):218. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040218
Chicago/Turabian StylePortillo-Torres, Lizbeth Anahí, Aurea Bernardino-Nicanor, Carlos Alberto Gómez-Aldapa, Simplicio González-Montiel, Esmeralda Rangel-Vargas, José Roberto Villagómez-Ibarra, Leopoldo González-Cruz, Humberto Cortés-López, and Javier Castro-Rosas. 2019. "Hibiscus Acid and Chromatographic Fractions from Hibiscus Sabdariffa Calyces: Antimicrobial Activity against Multidrug-Resistant Pathogenic Bacteria" Antibiotics 8, no. 4: 218. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040218