In Vitro Antibacterial Activity and Mechanism of Vanillic Acid against Carbapenem-Resistant Enterobacter cloacae
Abstract
:1. Introduction
2. Result
2.1. Minimum Inhibitory Concentration of VA against CREC
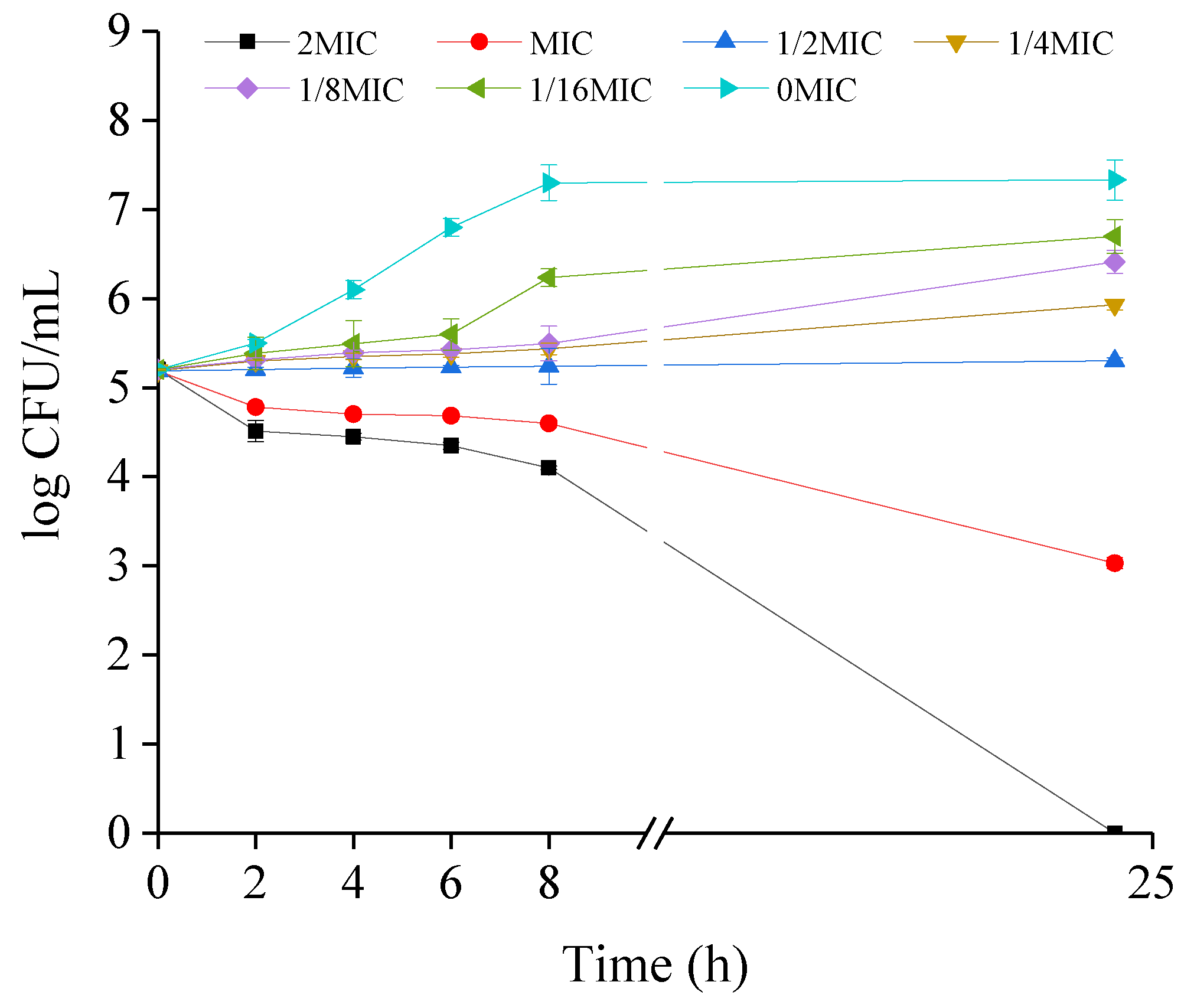
2.2. Growth Kinetics of CREC Cells in the Presence of VA
2.3. VA Exposure Results in the Decrease in Intracellular Adenosine Triphosphate (ATP)Concentrations, pH and Membrane Potential of CREC
2.4. VA Treatment Improves Cell Membrane Permeability of CREC
2.5. Changes in Cell Morphology of CREC Occurs in Response to VA
2.6. A Combination of Crystal Violet Staining, FESEM and Confocal Laser Scanning Microscopy Suggests the Inhibitory Effect of VA on Biofilm Formation of CREC
2.7. CLSM Images Display the Inactivation of VA against CREC Cells within Biofilms
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Bacterial and Culture Condition
4.3. Minimum Inhibitory Concentration (MIC) Determination
4.4. Growth Curves
4.5. Measurement of ATP Levels
4.6. Intracellular pH (pHin) Measurements
4.7. Membrane Potential Assay
4.8. Assessing Bacterial Membrane Integrity
4.9. Transmission Electron Microscopy
4.10. Field Emission Scanning Electron Microscopy
4.11. Biofilm Examination
4.12. Evaluation of Cell Damages within Biofilms
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brouwer, M.S.M.; Tehrani, K.; Rapallini, M.; Geurts, Y.; Kant, A.; Harders, F.; Mashayekhi, V.; Martin, N.I.; Bossers, A.; Mevius, D.J.; et al. Novel carbapenemases FLC-1 and IMI-2 encoded by an Enterobacter cloacae complex isolated from food products. Antimicrob. Agents Chemother. 2019, 63, 6. [Google Scholar]
- Esteban-Cuesta, I.; Dorn-In, S.; Drees, N.; Holzel, C.; Gottschalk, C.; Gareis, M.; Schwaiger, K. Antimicrobial resistance of Enterobacter cloacae complex isolates from the surface of muskmelons. Int. J. Food Microbiol. 2019, 301, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, H.; Liang, L.; Wang, G.; Xu, X.; Wang, H. Response of formed-biofilm of Enterobacter cloacae, Klebsiella oxytoca, and Citrobacter freundii to chlorite-based disinfectants. J. Food Sci. 2018, 83, 1326–1332. [Google Scholar] [CrossRef]
- Jiang, Y.; Jia, X.; Xia, Y. Risk factors with the development of infection with tigecycline- and carbapenem-resistant Enterobacter cloacae. Infect. Drug Resist. 2019, 12, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Janecko, N.; Martz, S.L.; Avery, B.P.; Daignault, D.; Desruisseau, A.; Boyd, D.; Irwin, R.J.; Mulvey, M.R.; Reid-Smith, R.J. Carbapenem-resistant enterobacter spp. in retail seafood imported from southeast Asia to Canada. Emerg. Infect Dis. 2016, 22, 1675–1677. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Hebraud, M.; Chorianopoulos, N.; Langsrud, S.; Moretro, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: Causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2014, 97, 298–309. [Google Scholar] [CrossRef]
- Stanely, P.; Rajakumar, S.; Dhanasekar, K. Protective effects of vanillic acid on electrocardiogram, lipid peroxidation, antioxidants, proinflammatory markers and histopathology in isoproterenol induced cardiotoxic rats. Eur. J. Pharmacol. 2011, 668, 233–240. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Konteles, S.; Kalogeropoulos, N.; Karathanos, V.T. Thermal oxidation of vanillin affects its antioxidant and antimicrobial properties. Food Chem. 2009, 114, 791–797. [Google Scholar] [CrossRef]
- Suknasang, S.; Teethaisong, Y.; Kabkhunthod, S.; Mingsiritom, N.; Eumkeb, G. Antibacterial activity of colistin is resurrected by Stephania suberosa Forman extract against colistin-resistant Enterobacter cloacae. Lett. App. Microbiol. 2019, 69, 128–135. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.C.; Abreu, R.M.V.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. App. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Liu, M.; Yang, K.; Wang, J.; Zhang, J.; Qi, Y.; Wei, X.; Fan, M. Young astringent persimmon tannin inhibits methicillin-resistant Staphylococcus aureus isolated from pork. LWT Food Sci. Technol. 2013, 100, 48–55. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Sun, Y.; Yang, M.; Song, K.; Zheng, Z.; Chen, Y.; Liu, X.; Jia, Z.; Dong, R. Antimicrobial activity of ferulic acid against Cronobacter sakazakii and possible mechanism of action. Foodborne Pathog. Dis. 2016, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jin, W.; Wang, J.; Sun, Y.; Wu, X.; Liu, L. Antibacterial and anti-biofilm activities of peppermint essential oil against Staphylococcus aureus. LWT Food Sci. Technol. 2019, 101, 639–645. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R.H. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage Microorganisms. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.; Garcia, S.; Heredia, N. Extracts of edible and medicinal plants damage membranes of Vibrio cholerae. Appl. Environ. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef]
- Na, S.; Kim, J.H.; Rhee, Y.K.; Oh, S.W. Enhancing the antimicrobial activity of ginseng against Bacillus cereus and Staphylococcus aureus by heat treatment. Food Sci. Biotechnol. 2018, 27, 203–210. [Google Scholar] [CrossRef]
- Matijasevic, D.; Pantic, M.; Raskovic, B.; Pavlovic, V.; Duvnjak, D.; Sknepnek, A.; Niksic, M. The antibacterial activity of Coriolus versicolor methanol extract and its effect on ultrastructural changes of Staphylococcus aureus and Salmonella Enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef]
- Jakobsen, T.H.; Tolker-Nielsen, T.; Givskov, M. Bacterial biofilm control by perturbation of bacterial signaling processes. Int. J. Mol. Sci. 2017, 18, 1970. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.R.; Yuan, D.D.; Yi, G.J.; Zhou, Y.Y.; Cheng, Q.; Zhu, J.; Li, M.X. Developing natural products as potential anti-biofilm agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Miriam, D.H.; Benjamin, S.; Yeshayahu, N. Bactericidal activity of the organo-tellurium compound AS101 against Enterobacter cloacae. J. Antimicrob. Chemother. 2012, 67, 2165–2172. [Google Scholar]
- Choi, H.A.; Cheong, D.E.; Lim, H.D.; Kim, W.H.; Ham, M.H.; Oh, M.H.; Wu, Y.; Shin, H.J.; Kim, G.J. Antimicrobial and anti-biofilm activities of the methanol extracts of medicinal plants against dental pathogens Streptococcus mutans and Candida albicans. J. Microbiol. Biotechnol. 2017, 27, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 2017, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Sianglum, W.; Saeloh, D.; Tongtawe, P.; Wootipoom, N.; Indrawattana, N.; Voravuthikunchai, S.P. Early effects of rhodomyrtone on membrane integrity in methicillin-resistant Staphylococcus aureus. Microb. Drug Resist. 2018, 24, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, M.; Yang, J.; Chen, L.; Yu, P.; Wang, J. In vitro antibacterial activity and mechanism of monocaprylin against Escherichia coli and Staphylococcus aureus. J. Food Prot. 2018, 81, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, F.; Du, L.; Zhao, T.; Doyle, M.; Wang, D.; Zhang, X.; Sun, Z.; Xu, W. Antibacterial and antibiofilm activity of phenyllactic acid against Enterobacter cloacae. Food Control 2018, 84, 442–448. [Google Scholar] [CrossRef]
- Joung, D.K.; Mun, S.H.; Choi, S.H.; Kang, O.H.; Kim, S.B.; Lee, Y.S.; Zhou, T.; Kong, R.; Choi, J.G.; Shin, D.W.; et al. Antibacterial activity of oxyresveratrol against methicillin-resistant Staphylococcus aureus and its mechanism. Exp. Ther. Med. 2016, 12, 1579–1584. [Google Scholar] [CrossRef]
- Shi, C.; Che, M.; Zhang, X.; Liu, Z.; Meng, R.; Bu, X.; Ye, H.; Guo, N. Antibacterial activity and mode of action of totarol against Staphylococcus aureus in carrot juice. J. Food Sci. Technol. 2018, 55, 924–934. [Google Scholar] [CrossRef]
- Dasagrandhi, C.; Park, S.; Jung, W.K.; Kim, Y.M. Antibacterial and biofilm modulating potential of ferulic acid-grafted chitosan against human pathogenic bacteria. Int. J. Mol. Sci. 2018, 19, 2157. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Liu, S.; Song, F.; Guo, J.; Huang, C. Influence of naringenin on the biofilm formation of Streptococcus mutans. J. Dent. 2018, 76, 24–31. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Nynca, A.; Bialobrzewski, I.; Kocot, A.M.; Laguna, J. Assessment of the bacterial viability of chlorine- and quaternary ammonium compounds-treated Lactobacillus cells via a multi-method approach. J. Appl. Microbiol. 2019, 126, 1070–1080. [Google Scholar] [CrossRef]







| Strain | Carbapenemase Genes | MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| VA | IMP | MEM | PIP/TAZ | CTX | CAZ | Polymyxin B | ||
| CREC-3 | NDM-1 | 600 | 32 | 32 | >256 | >256 | >128 | 0.25 |
| CREC-7 | IMP | 600 | 16 | 32 | >128 | >256 | >128 | 0.25 |
| CREC-16 | KPC, NDM-1 | 600 | 32 | 64 | >256 | >256 | >256 | 0.25 |
| CREC-22 | KPC | 600 | 16 | 64 | >256 | >256 | >128 | 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, W.; Fu, Y.; Liu, M.; Wang, T.; Zhang, J.; Yang, M.; Sun, Z.; Li, X.; Li, Y. In Vitro Antibacterial Activity and Mechanism of Vanillic Acid against Carbapenem-Resistant Enterobacter cloacae. Antibiotics 2019, 8, 220. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040220
Qian W, Fu Y, Liu M, Wang T, Zhang J, Yang M, Sun Z, Li X, Li Y. In Vitro Antibacterial Activity and Mechanism of Vanillic Acid against Carbapenem-Resistant Enterobacter cloacae. Antibiotics. 2019; 8(4):220. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040220
Chicago/Turabian StyleQian, Weidong, Yuting Fu, Miao Liu, Ting Wang, Jianing Zhang, Min Yang, Zhaohuan Sun, Xiang Li, and Yongdong Li. 2019. "In Vitro Antibacterial Activity and Mechanism of Vanillic Acid against Carbapenem-Resistant Enterobacter cloacae" Antibiotics 8, no. 4: 220. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040220





