Photoinduced Antibacterial Activity of the Essential Oils from Eugenia brasiliensis Lam and Piper mosenii C. DC. by Blue Led Light
Abstract
:1. Introduction
2. Results
2.1. Chemical Profile of the Essential Oils
2.2. Antibacterial Activity by Direct Contact
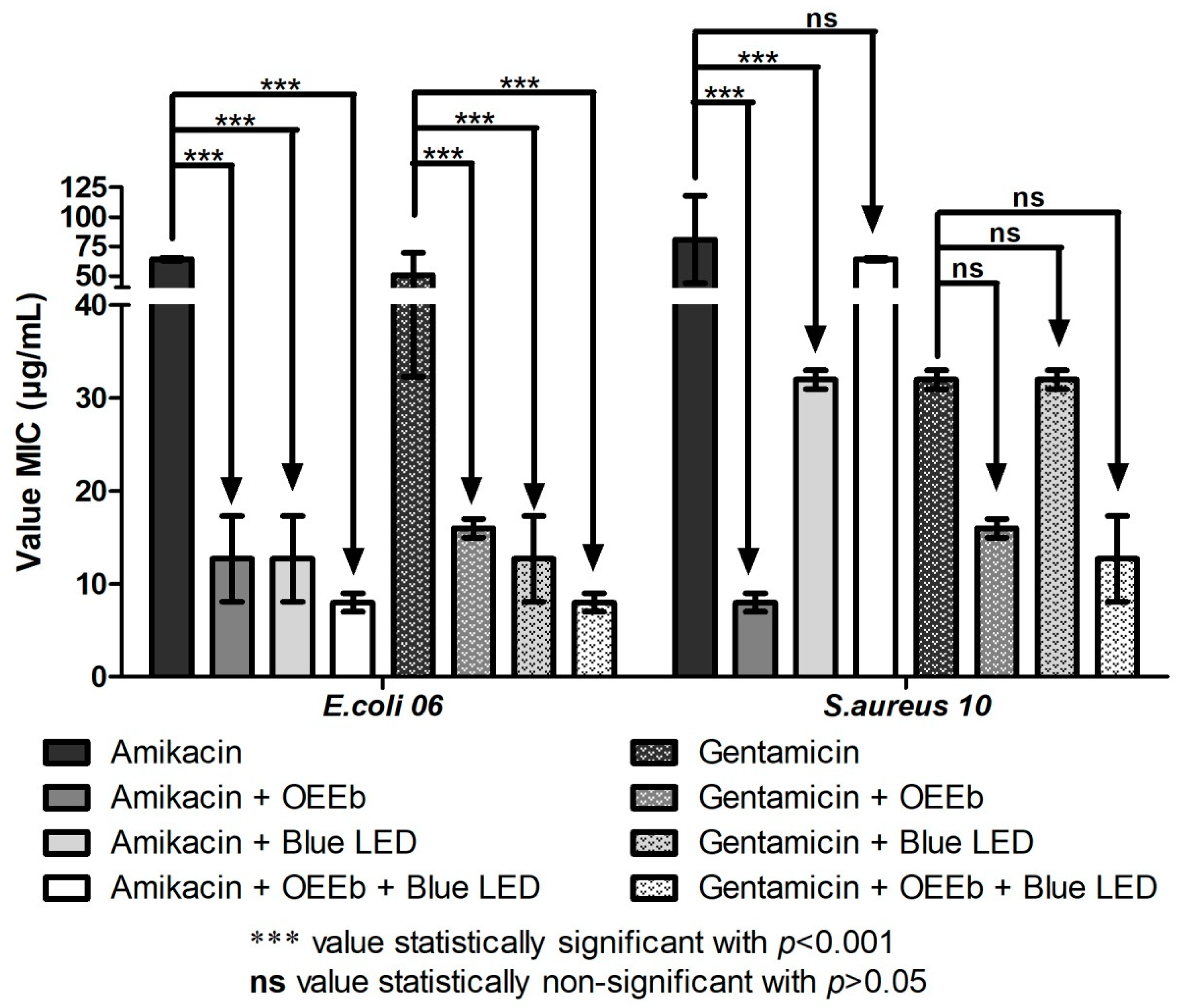
2.3. Modulating Effect of Essential Oils in Association with Blue LED Light on the Activity of Aminoglycosides
3. Methodology
3.1. Plant Material and Extraction of Essential Oils
3.2. Determination of the Chemical Composition of the Essential Oils
3.3. Antibiotics, Culture Media and Microorganisms
3.4. Preparation of Test Solutions
3.5. Determination of the Minimum Inhibitory Concentration (MIC) by Direct Contact
3.6. Evaluation of Modulating Activity Associated with Blue LED Light Exposure
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sampaio, P.S.; Sancho, L.G.; Lago, R.F. Implementação da nova regulamentação para prescrição e dispensação de antimicrobianos: Possibilidades e desafios. Cad. Saúde Colet. 2018, 26, 15–22. [Google Scholar] [CrossRef]
- Nathan, C.; Cars, O. Antibiotic resistance-problems, progress, and prospects. N. Engl. J. Med. 2014, 371, 1761–1763. [Google Scholar] [CrossRef] [PubMed]
- Piltcher, O.B.; Kosugi, E.M.; Sakano, E.; Mion, O.; Testa, J.R.G.; Romano, F.R.; Santos, M.C.J.; Di Francesco, R.C.; Mitre, E.I.; Bezerra, T.F.P.; et al. How to avoid the inappropriate use of antibiotics in upper respiratory tract infections? A position statement from an expert panel. Braz. J. Otorhinolaryngol. 2018, 84, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.R.; Shrivastava, P.S.; Ramasamy, J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018, 32, 76. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.B.; Boerger, M.R.T.; Negrelle, R.B.; Bergo, C. Effect of ultrasound and infrared drying methods on quantitative and qualitative characteristics of Satureja bachtiarica essential oil. J. Essent. Oil-Bear. Plants 2017, 20, 1196–1208. [Google Scholar]
- Souza, M.R.; Maingredy, X.; Milton, B.; Dryelle, S.P.; Júnior, M. Fioterápicos no tratamento de transtornos de ansiedade. Eletron. J. Pharm. 2016, 13, 11–12. [Google Scholar]
- Sobrinho, T.J.S.P.; Castro, V.T.N.A.; Saraiva, A.M.; Almeida, D.M.; Tavares, E.A.; Amorim, E.L.C. Phenolic content and antioxidant capacity of our Cnidoscolus species (Euphorbiaceae) used as ethnopharmacologicals in Caatinga. Afr. J. Pharm. Pharmacol. 2011, 5, 2310–2316. [Google Scholar]
- Coutinho, H.D.M.; Costa, J.G.M.; Siqueira-Júnior, J.P.; Falcão-Silva, V.; Lima, E. Fruits to potentiate the antibiotic activity: The effect of Eugenia uniflora and Eugenia jambolanum L. against MRSA. Acta Aliment. 2012, 41, 67–72. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de identificação e cultivo de plantas arbóreas nativas do Brasil; Plantarum: Nova Odessa, Brazil, 2002. [Google Scholar]
- Benfatti, C.S.; Cordova, S.M.D.; Guedes, A.; Magina, M.D.A.; Cordova, C.M.M.D. Atividade antibacteriana in vitro de extratos brutos de espécies de Eugenia sp frente a cepas de molicutes. Rev. Pan-Amaz. Saúde. 2010, 1, 33–39. [Google Scholar]
- Suguino, E.; Martins, A.N.; Minami, K.; Narita, N.; Perdoná, M.J. Efeito da porosidade do substrato casca de Pínus no desenvolvimento de mudas de Grumixameira. Rev. Bras. Frut. Espec. 2011, 33, 643–648. [Google Scholar] [CrossRef]
- Magina, M.D.A.; Dalmarco, E.M.; Dalmarco, J.B.; Colla, G.; Pizzolatti, M.G.; Brighente, I.M.C. Bioactive triterpenes and phenolics of leaves of Eugenia brasiliensis. Quim. Nova 2012, 35, 1184–1188. [Google Scholar] [CrossRef]
- BFG-The Brazil Flora Group. Growing knowledge: An overview of seed plant diversity in Brazil. Rodriguésia 2015, 66, 1085–1113. [Google Scholar] [CrossRef]
- Christ, J.A.; Hollunder, R.K.; Carvalho, M.S.; Ferreira, M.F.D.S.; Garbin, M.L.; Carrijo, T.T. DNA fingerprinting based on SSR amplification profiles for Piper species identification (Piperaceae). Acta Bot. Bras. 2018, 32, 511–520. [Google Scholar] [CrossRef]
- Gogosz, A.M.; Boerger, M.R.T.; Negrelle, R.B.; Bergo, C. Anatomia foliar comparativa de nove espécies do gênero Piper (Piperaceae). Rodriguésia 2012, 63, 405–417. [Google Scholar] [CrossRef]
- Bernuci, K.; Iwanaga, C.C.; Fernandez-Andrade, C.M.M.; Lorenzetti, F.B.; Torres-Santos, E.C.; Faiões, V.D.S.; Gonçalves, J.E.; Amaral, W.; Deschamps, C.; Scodro, R.B.L.; et al. Evaluation of chemical composition and antileishmanial and antituberculosis activities of essential oils of Piper species. Molecules 2016, 21, 1698. [Google Scholar] [CrossRef]
- Velandia, S.A.; Quintero, E.; Stashenko, E.E.; Ocazionez, R.E. Actividad antiproliferativa de aceites esenciales de plantas cultivadas en Colombia. Acta Biol. Colomb. 2018, 23, 89–198. [Google Scholar] [CrossRef]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Visible light-induced killing of bacteria as a function of wavelength: Implication for wound healing. Lasers Surg. Med. 2010, 42, 467–472. [Google Scholar] [CrossRef]
- Dourado, K.B.V.; Carnevali-Junior, L.C.; Paulo, R.J.F.; Gomes, A.C. LEDTERAPIA, uma nova perspectiva terapêutica ao tratamento de doenças da pele, cicatrização de feridas e reparação tecidual. Ensaios e Ciência: Ciências agrárias, biológicas e da saúde 2011, 15, 231–248. [Google Scholar]
- Apel, M.A.; Sobral, M.; Schapoval, E.E.S.; Henriques, A.T.; Menut, C.; Bessiere, J.M. Essential oils from Eugenia species. Part VII: Sections Phyllocalyx and Stenocalyx. J. Essent. Oil Res. 2004, 16, 135–138. [Google Scholar] [CrossRef]
- Fischer, D.C.H.; Limberger, R.P.; Henriques, A.T.; Moreno, P.R. Essential oils from leaves of two Eugenia brasiliensis specimens from Southeastern Brazil. J. Essent. Oil Res. 2005, 17, 499–500. [Google Scholar] [CrossRef]
- Moreno, P.R.H.; Lima, M.E.L.; Sobral, M.; Young, M.C.M.; Cordeiro, I.; Apel, M.A.; Limberger, R.P.; Henriques, A.T. Essential oil composition of fruit colour varieties of Eugenia brasiliensis Lam. Sci. Agric. 2007, 64, 428–432. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-Caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Cardoso, M.G.; Batista, L.R.; Mallet, A.C.T.; Machado, S.M.F. Óleos essenciais de Cymbopogon nardus, Cinnamomum zeylanicum e Zingiber officinale: Composição, atividades antioxidante e antibacteriana. Rev. Ciên. Agron. 2012, 43, 399–408. [Google Scholar] [CrossRef]
- Radünz, L.L.; Melo, E.C.; Barbosa, L.C.A.; Rocha, R.P.; Berbert, P.A. Rendimento extrativo de cumarina de folhas de guaco (Mikania glomerata Sprengel) submetidas a diferentes temperaturas de secagem. Rev. Bras. Plantas Med. 2012, 14, 453–457. [Google Scholar] [CrossRef]
- Magina, M.D.A.; Dalmarco, E.M.; Wisniewski Jr, A.; Simionatto, E.L.; Dalmarco, J.B.; Pizzolatti, M.G.; Brighente, I.M.C. Chemical composition and antibacterial activity of essential oils of Eugenia species. J. Nat. Med. 2009, 63, 345–350. [Google Scholar] [CrossRef]
- Alves, H.S.; Rocha, W.R.V.; Fernandes, A.F.C.; Nunes, L.E.; Pinto, D.S.; Costa, J.I.V.; Chaves, M.C.O.; Catão, R.M.R. Actividad antimicrobiana de productos obtenidos a partir de especies de Piper (Piperaceae). Rev. Cuba. Plant. Med. 2016, 21, 168–180. [Google Scholar]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Sieniawska, E.; Sawicki, R.; Swatko-Ossor, M.; Napiorkowska, A.; Przekora, A.; Ginalska, G.; Augustynowicz-Kopec, E. The effect of combining natural terpenes and antituberculous agents against reference and clinical Mycobacterium tuberculosis strains. Molecules 2018, 23, 176. [Google Scholar] [CrossRef]
- Silva, A.C.R.; Lopes, P.M.; Azevedo, M.M.B.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-Pinene and β-Pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods. Int. J. Food Microbiol 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Pereira, N.L.F.; Aquino, P.E.A.; Júnior, J.G.A.S.; Cristo, J.S.M.A.V.; Moura, F.F.; Ferreira, N.M.N.; Silva, M.K.N.; Nascimento, E.M.; Correia, F.M.A.; Cunha, F.A.B.; et al. Antibacterial activity and antibiotic modulating potential of the essential oil obtained from Eugenia jambolana in association with led lights. J. Photochem. Photobiol. B 2017, 174, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Matias, E.F.F.; Bezerra, V.B.; Barros, R.O.; Lucena, A.L.V.M.; Leite, V.D.; Feitosa, J.H.F.; Silva, M.K.N. Avaliação da atividade antibacteriana e moduladora do óleo essencial de Cordia verbenacea DC. associado às luzes de LED. Rev. Interfaces 2017, 5, 7–14. [Google Scholar] [CrossRef]
- Caffarel-Salvador, E.; Kearney, M.C.; Mairs, R.; Gallo, L.; Stewart, S.A.; Brady, A.J.; Donnelly, R.F. Methylene blue-loaded dissolving microneedles: Potential use in photodynamic antimicrobial chemotherapy of infected wounds. Pharmaceutics 2015, 7, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; Cristofolinli, G.M.A.F. Led terapia na faixa do vermelho ao infravermelho: Uma nova abordagem sob a visão quântica para a saúde. Rev. Saúde Quântica 2014, 3, 102–110. [Google Scholar]
- Brito, S.V.; Ferreira, F.S.; Siqueira-Júnior, J.P.; Costa, J.G.; Almeida, W.O.; Coutinho, H.D. Phototoxic and modulatory effects of natural products from the skin of Rhinella jimi (Stevaux, 2002). Rev. Bras. Farmacogn. 2012, 22, 82–87. [Google Scholar] [CrossRef]
- Coutinho, H.D.M.; Costa, J.G.M.; Siqueira-Júnior, J.P.; Lima, E.O. In vitro phototoxic activity of Eugenia jambolana L. and Hyptis martiusii Benth. J. Photochem Photobiol. B 2009, 96, 63–65. [Google Scholar] [CrossRef]
- Ragàs, X.; He, X.; Agut, M.; Roxo-Rosa, M.; Gonsalves, A.R.; Serra, A.C.; Nonell, S. Singlet oxygen in antimicrobial photodynamic therapy: Photosensitizer-dependent production and decay in E. coli. Molecules 2013, 18, 2712–2725. [Google Scholar] [CrossRef]
- Figueiredo, F.G.; Ferreira, E.O.; Lucena, B.F.F.; Torres, C.M.G.; Lucetti, D.L.; Lucetti, E.C.P.; Silva, J.M.F.L.; Santos, F.A.V.; Medeiros, C.R.; Oliveira, G.M.M.; et al. Modulation of the antibiotic activity by extracts from Amburana cearensis A. C. Smith and Anadenanthera macrocarpa (Benth.) Brenan. Biomed. Res. Int. 2013, 2013, 1–6. [Google Scholar]
- Wasicky, R. Uma modificação do aparelho de Clevenger para extração de óleos essenciais. Revista da Faculdade Farmácia e Bioquímica 1963, 1, 77–81. [Google Scholar]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Publ. Corp. 2007, 18, 803–806. [Google Scholar]
- NCCLS. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, 6th ed.; NCCLS document M7-A6; NCCLS: Wayne, PA, USA, 2003. [Google Scholar]
- Coutinho, H.D.M.; Costa, J.G.M.; Siqueira-Júnior, J.P.; Lima, E.O. In Vitro anti-staphylococcal activity of Hyptis martiusii Benth against methicillin-resistant Staphylococcus aureus: MRSA strains. Rev. Bras. Farmacogn. 2008, 18, 670–675. [Google Scholar] [CrossRef] [Green Version]


| RI Calculated | Composition | % |
|---|---|---|
| 935 | tricyclene | 7.27 |
| 980 | sabinene | 5.39 |
| 1030 | limonene | 8.96 |
| 1034 | 1,8-cineole | 4.84 |
| 1103 | Linalool | 1.54 |
| 1196 | α-terpineol | 1.53 |
| 1425 | (E)-caryophyllene | 2.69 |
| 1503 | β-macrocarpene | 2.30 |
| 1521 | γ-cadinene | 1.22 |
| 1530 | δ-cadinene | 5.26 |
| 1576 | longipinanol | 1.01 |
| 1587 | spathulenol | 6.16 |
| 1593 | thujopsan-2-α-ol | 6.11 |
| 1603 | cubeban-11-ol | 4.40 |
| 1613 | 5-epi-7-epi-α-eudesmol | 2.12 |
| 1624 | 1,10-di-epi-cubenol | 1.23 |
| 1638 | 1-epi-cubenol | 4.35 |
| 1651 | α-muurolol (=torreyol) | 12.01 |
| 1657 | valerianol | 3.60 |
| 1665 | selin-11-em-4-α-ol | 7.10 |
| RI Calculated | Composition | % |
|---|---|---|
| 935 | α-pinene | 14.59 |
| 980 | β-pinene | 2.72 |
| 1445 | aromadendrene | 2.12 |
| 1460 | α-humulene | 3.85 |
| 1468 | allo aromadendrene | 3.74 |
| 1483 | germacrene D | 1.08 |
| 1487 | γ-muurolene | 2.77 |
| 1493 | β-selinene | 1.92 |
| 1504 | bicyclogermacrene | 12.25 |
| 1531 | δ-cadinene | 4.74 |
| 1587 | fokienol | 8.43 |
| 1592 | globulol | 6.15 |
| 1613 | sesquithuriferol | 1.5 |
| 1619 | epi-cedrol | 1.89 |
| 1652 | α-cadinol | 2.02 |
| 1665 | neo-intermedeol | 1.62 |
| 1670 | intermedeol | 1.66 |
| 1695 | caryophyllene acetate | 4.73 |
| Bacterial Strains | OEEb | EOPm | ||
|---|---|---|---|---|
| Normal light | Blue LED Light | Normal light | Blue LED Light | |
| SA ATCC 25923 | ≥1024 | ≥1024 | 512 | 512 |
| SA 10 | ≥1024 | ≥1024 | 512 | 512 |
| EC ATCC 25922 | ≥1024 | ≥1024 | ≥1024 | ≥1024 |
| EC 06 | ≥1024 | ≥1024 | ≥1024 | ≥1024 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macedo da Silva, R.O.; Gonçalves Castro, J.W.; de Menezes Dantas Junior, O.; Justino de Araújo, A.C.; do Nascimento Silva Leandro, M.K.; Oliveira Costa, R.J.; Leite Pinto, L.; Garcia Leandro, L.M.; Silva, L.E.d.; do Amaral, W.; et al. Photoinduced Antibacterial Activity of the Essential Oils from Eugenia brasiliensis Lam and Piper mosenii C. DC. by Blue Led Light. Antibiotics 2019, 8, 242. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040242
Macedo da Silva RO, Gonçalves Castro JW, de Menezes Dantas Junior O, Justino de Araújo AC, do Nascimento Silva Leandro MK, Oliveira Costa RJ, Leite Pinto L, Garcia Leandro LM, Silva LEd, do Amaral W, et al. Photoinduced Antibacterial Activity of the Essential Oils from Eugenia brasiliensis Lam and Piper mosenii C. DC. by Blue Led Light. Antibiotics. 2019; 8(4):242. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040242
Chicago/Turabian StyleMacedo da Silva, Rakel Olinda, José Walber Gonçalves Castro, Orlando de Menezes Dantas Junior, Ana Carolina Justino de Araújo, Maria Karollyna do Nascimento Silva Leandro, Raíra Justino Oliveira Costa, Luciely Leite Pinto, Lívia Maria Garcia Leandro, Luiz E. da Silva, Wanderlei do Amaral, and et al. 2019. "Photoinduced Antibacterial Activity of the Essential Oils from Eugenia brasiliensis Lam and Piper mosenii C. DC. by Blue Led Light" Antibiotics 8, no. 4: 242. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040242







