Chronic Lyme Disease: An Evidence-Based Definition by the ILADS Working Group †
Abstract
:1. Introduction
2. Chronic Lyme Disease Definition
3. Microbiology
4. Vector
5. Pathophysiologic Basis of Chronic Lyme Disease
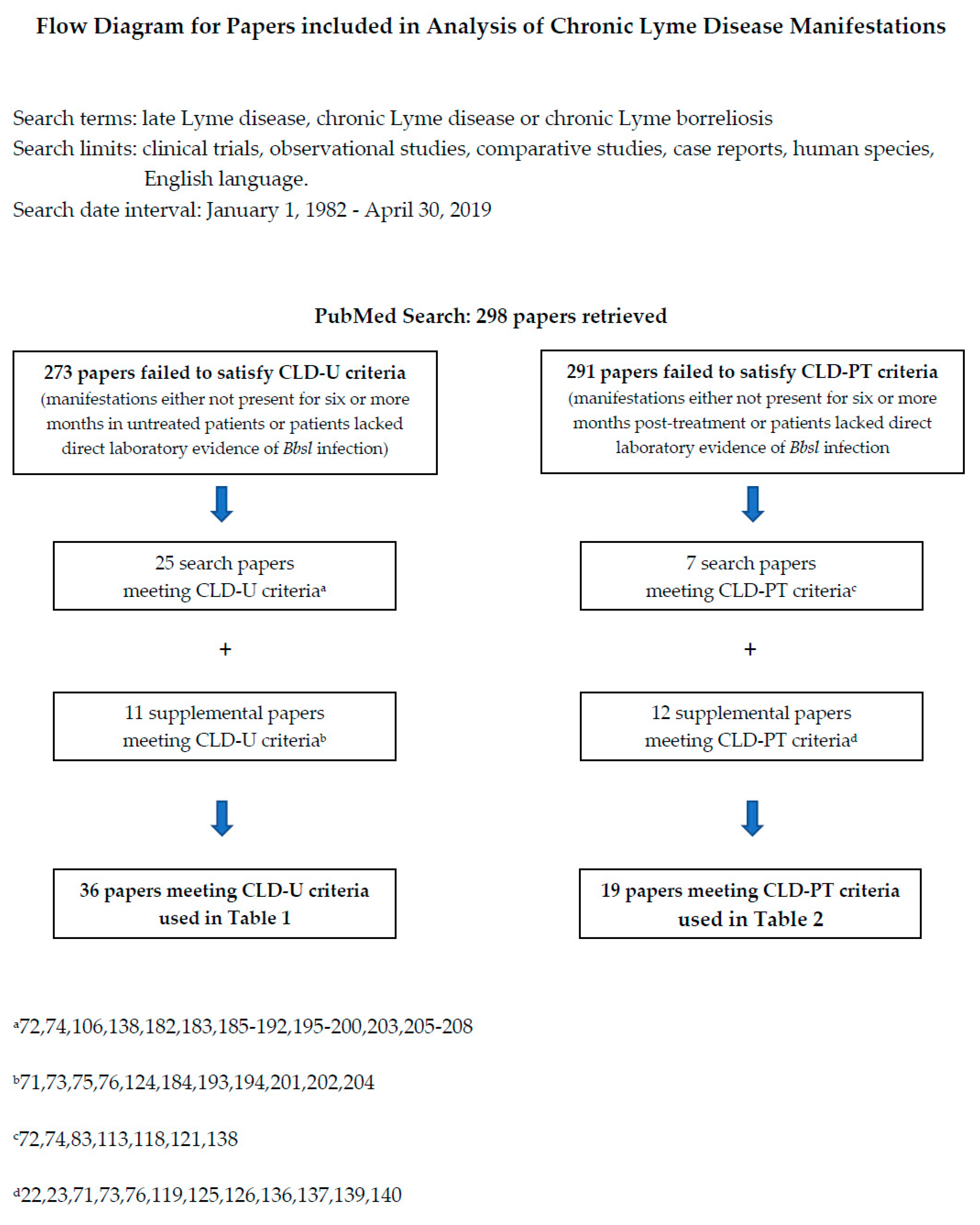
6. Clinical Manifestations of Chronic Lyme Disease
Methods
7. Comparison to the Definition of Post-Treatment Lyme Disease Syndrome
8. Limitations
9. Conclusions: Summary and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Bb | Borrelia burgdorferi |
| Bbsl | Borrelia burgdorferi sensu lato |
| CDC | Centers for Disease Control |
| CLD | Chronic Lyme disease |
| CLD-U | Chronic Lyme disease, untreated |
| CLD-PT | Chronic Lyme disease, previously treated |
| CLD-T | chronic Lyme disease, treated |
| CSF | cerebrospinal fluid |
| EM | erythema migrans |
| DNA | deoxyribonucleic acid |
| HEENT | head, ears, eyes, nose and throat |
| IgM | immunoglobulin M |
| IgG | immunoglobulin G |
| ILADS | International Lyme and Associated Diseases Society |
| OSP | outer surface protein |
| PCR | polymerase chain reaction |
| PLDS | Post Lyme disease Syndrome |
| PTLDS | Post-Treatment Lyme disease Syndrome |
| QoL | Quality of Life |
| TBD | tick-borne disease |
Appendix A
| Most Commonly Reported Bbsl Pathogens | ||
| Genospecies | Evidence | Selected References |
| B. afzelii | Culture | Strle (2006) [37] |
| B. burgdorferi sensu stricto | Culture | Steere (1984) [34] Nowakowski (2009) [35] Smith (2002) [36] |
| B. garinii | Culture | Strle (2006) [37] |
| Less Commonly Reported Bbsl Pathogens | ||
| B. americana | PCR | Clark (2013) [50] |
| B. andersonii | PCR | Clark (2013) [50] |
| B. bavariensis | PCR | Markowicz (2015) [48] Tijsse-Klasen (2013) [49] |
| B. bissettii | PCR | Golovchenko (2016) [45] Rudenko (2008) [46] Rudenko (2009) [47] |
| B. lusitaniae | PCR | Collares-Pereira (2004) [44] |
| B. mayonii | PCR | Pritt (2016) [43] |
| B. spielmanii | Culture & PCR | Maraspin (2006) [38] |
| PCR | Fingerle (2008) [39] Földvári (2005) [40] | |
| B. valaisiana | Immunoblot | Ryffel (1999) [41] |
| B. sp A14S | PCR | Wang (1999) [42] |
| Bbsl Genospecies Without Established Pathogenicity | ||
| B. californiensis | Postic (2007) [54] | |
| B. carolinensis | Foley (2014) [55] | |
| B. japonica | Rudenko (2011) [51] | |
| B. kurtenbachii | Margos (2010) [56] | |
| B. lanei | Margos (2017) [57] | |
| B. sinica | Rudenko (2011) [51] | |
| B. tanuki | Rudenko (2011) [51] | |
| B. turdi | Rudenko (2011) [51] | |
| B. yangtze | Rudenko (2011) [51] | |
References
- Schwartz, A.M.; Hinckley, A.F.; Mead, P.S.; Hook, S.A.; Kugeler, K.J. Surveillance for Lyme Disease-United States, 2008–2015. MMWR Surveill. Summ. 2017, 66, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.S.; Kirstein, F.; Robertson, J.N.; Stein, J.; Kahl, O. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks and rodents in a recreational park in south-western Ireland. Exp. Appl. Acarol. 1999, 23, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; Delorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Logigian, E.L.; Kaplan, R.F.; Steere, A.C. Chronic neurologic manifestations of Lyme disease. N. Engl. J. Med. 1990, 323, 1438–1444. [Google Scholar] [CrossRef]
- Luft, B.J.; Gorevic, P.D.; Halperin, J.J.; Volkman, D.J.; Dattwyler, R.J. A perspective on the treatment of Lyme borreliosis. Rev. Infect. Dis. 1989, 11, S1518–S1525. [Google Scholar] [CrossRef] [Green Version]
- Steere, A.C.; Malawista, S.E.; Bartenhagen, N.H.; Spieler, P.N.; Newman, J.H.; Rahn, D.W.; Hutchinson, G.J.; Green, J.; Snydman, D.R.; Taylor, E. The clinical spectrum and treatment of Lyme disease. Yale J. Biol. Med. 1984, 57, 453–461. [Google Scholar]
- Berger, B.W. Dermatologic manifestations of Lyme disease. Rev. Infect. Dis. 1989, 11, S1475–S1481. [Google Scholar] [CrossRef]
- Rauer, S.; Kastenbauer, S.; Fingerle, V.; Hunfeld, K.P.; Huppertz, H.I.; Dersch, R. Lyme Neuroborreliosis. Dtsch. Arztebl. Int. 2018, 115, 751–756. [Google Scholar] [CrossRef]
- Maine Department of Health and Human Services, Center for Disease Control and Prevention, Division of Disease Surveillance. Infectious Disease Epidemiology Program, 2019, Report to Maine Legislature: Lyme and Other Tickborne Illnesses. Available online: https://www.maine.gov/dhhs/mecdc/infectious-disease/epi/vector-borne/lyme/#reports (accessed on 26 June 2019).
- Asch, E.S.; Bujak, D.I.; Weiss, M.; Peterson, M.G.; Weinstein, A. Lyme disease: An infectious and postinfectious syndrome. J. Rheumatol. 1994, 21, 454–461. [Google Scholar]
- Aucott, J.N.; Rebman, A.W.; Crowder, L.A.; Kortte, K.B. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: Is there something here? Qual. Life Res. 2013, 22, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Cameron, D.J.; Johnson, L.B.; Maloney, E.L. Evidence assessments and guideline recommendations in Lyme disease: The clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev. Anti Infect. Ther. 2014, 12, 1103–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logigian, E.L.; Kaplan, R.F.; Steere, A.C. Successful treatment of Lyme encephalopathy with intravenous ceftriaxone. J. Infect. Dis. 1999, 180, 377–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luft, B.J.; Dattwyler, R.J.; Johnson, R.C.; Luger, S.W.; Bosler, E.M.; Rahn, D.W.; Masters, E.J.; Grunwaldt, E.; Gadgil, S.D. Azithromycin compared with amoxicillin in the treatment of erythema migrans. A double-blind, randomized, controlled trial. Ann. Intern. Med. 1996, 124, 785–791. [Google Scholar] [CrossRef]
- Shadick, N.A.; Phillips, C.B.; Logigian, E.L.; Steere, A.C.; Kaplan, R.F.; Berardi, V.P.; Duray, P.H.; Larson, M.G.; Wright, E.A.; Ginsburg, K.S.; et al. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann. Intern. Med. 1994, 121, 560–567. [Google Scholar] [CrossRef]
- Fallon, B.A.; Keilp, J.G.; Corbera, K.M.; Petkova, E.; Britton, C.B.; Dwyer, E.; Slavov, I.; Cheng, J.; Dobkin, J.; Nelson, D.R.; et al. A randomized, placebo-controlled trial of repeated IV antibiotic therapy for Lyme encephalopathy. Neurology 2008, 70, 992–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klempner, M.S.; Hu, L.T.; Evans, J.; Schmid, C.; Johnson, G.; Trevino, R.; Norton, D.; Levy, L.; Wall, D.; McCall, J.; et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N. Engl. J. Med. 2001, 345, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Krupp, L.B.; Hyman, L.G.; Grimson, R.; Coyle, P.K.; Melville, P.; Ahnn, S.; Dattwyler, R.; Chandler, B. Study and treatment of post Lyme disease (STOP-LD): A randomized double masked clinical trial. Neurology 2003, 60, 1923–1930. [Google Scholar] [CrossRef]
- Cameron, D. Severity of Lyme disease with persistent symptoms. Insights from a double-blind placebo-controlled clinical trial. Minerva Med. 2008, 99, 489–496. [Google Scholar]
- Johnson, L.; Wilcox, S.; Mankoff, J.; Stricker, R.B. Severity of chronic Lyme disease compared to other chronic conditions: A quality of life survey. PeerJ 2014, 27, e322. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, E.; Szpak, G.M.; Piłkowska, E.; Habib, N.; Lipczyńska-Lojkowska, W.; Rudnicka, A.; Tylewska-Wierzbanowska, S.; Kulczycki, J. Central nervous system infection caused by Borrelia burgdorferi. Clinico-pathological correlation of three post-mortem cases. Folia Neuropathol. 1999, 37, 43–51. [Google Scholar] [PubMed]
- Cassarino, D.S.; Quezado, M.M.; Ghatak, N.R.; Duray, P.H. Lyme-associated parkinsonism: A neuropathologic case study and review of the literature. Arch. Pathol. Lab. Med. 2003, 127, 1204–1206. [Google Scholar] [PubMed]
- Liegner, K.B.; Duray, P.; Agricola, M.; Rosenkilde, C.; Yannuzzi, L.; Ziska, M.; Tilton, R.C.; Hulinska, D.; Hubbard, J.; Fallon, B.A. Lyme Disease and the Clinical Spectrum of Antibiotic-Responsive Chronic Meningoencephalomyelitides. J. Spirochetal Tick Borne Dis. 1997, 4, 61–73. [Google Scholar]
- Bransfield, R.C. Suicide and Lyme and associated diseases. Neuropsychiatr. Dis. Treat. 2017, 13, 1575–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.; Aylward, A.; Stricker, R.B. Healthcare access and burden of care for patients with Lyme disease: A large United States survey. Health Policy 2011, 102, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Adrion, E.R.; Aucott, J.; Lemke, K.W.; Weiner, J.P. Health care costs, utilization and patterns of care following Lyme disease. PLoS ONE 2015, 10, e0116767. [Google Scholar] [CrossRef]
- Stricker, R.B.; Fesler, M.F. Chronic Lyme Disease: A Working Case Definition. Am. J. Infect. Dis. 2018, 14, 1–14. [Google Scholar] [CrossRef]
- Horowitz, R.I.; Freeman, P.R. Precision Medicine: The Role of the MSIDS Model in Defining, Diagnosing, and Treating Chronic Lyme Disease/Post Treatment Lyme Disease Syndrome and Other Chronic Illness: Part 2. Healthcare 2018, 6, 129. [Google Scholar] [CrossRef] [Green Version]
- CSTE Position Statement 11-ID-04. Hepatitis B, Chronic 2012 Case Definition. Available online: https://wwwn.cdc.gov/nndss/conditions/hepatitis-b-chronic/case-definition/2012/ (accessed on 21 April 2017).
- Pressler, T.; Bohmova, C.; Conway, S.; Dumcius, S.; Hjelte, L.; Høiby, N.; Kollberg, H.; Tümmler, B.; Vavrova, V. Chronic Pseudomonas aeruginosa infection definition: EuroCareCF Working Group report. J. Cystic Fibrosis 2011, 10, S75–S78. [Google Scholar] [CrossRef] [Green Version]
- Krause, P.J.; Telford, S.R., III; Spielman, A.; Sikand, V.; Ryan, R.; Christianson, D.; Burke, G.; Brassard, P.; Pollack, R.; Peck, J.; et al. Concurrent Lyme disease and babesiosis: Evidence for increased severity and duration of illness. JAMA 1996, 275, 1657–1660. [Google Scholar] [CrossRef]
- Thompson, C.; Spielman, A.; Krause, P.J. Coinfecting deer-associated zoonoses: Lyme disease, babesiosis, and ehrlichiosis. Clin. Infect. Dis. 2001, 33, 676–685. [Google Scholar] [CrossRef] [Green Version]
- Aucott, J.N.; Crowder, L.A.; Kortte, K.B. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int. J. Infect. Dis. 2013, 17, e443–e449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steere, A.C.; Grodzicki, R.L.; Craft, J.E.; Shrestha, M.; Kornblatt, A.N.; Malawista, S.E. Recovery of Lyme disease spirochetes from patients. Yale J. Biol. Med. 1984, 57, 557–560. [Google Scholar] [PubMed]
- Nowakowski, J.; McKenna, D.; Nadelman, R.B.; Bittker, S.; Cooper, D.; Pavia, C.; Holmgren, D.; Visintainer, P.; Wormser, G.P. Blood cultures for patients with extracutaneous manifestations of Lyme disease in the United States. Clin. Infect. Dis. 2009, 49, 1733–1735. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.P.; Schoen, R.T.; Rahn, D.W.; Sikand, V.K.; Nowakowski, J.; Parenti, D.L.; Holman, M.S.; Persing, D.H.; Steere, A.C. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann. Intern. Med. 2002, 136, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Strle, F.; Ruzić-Sabljić, E.; Cimperman, J.; Lotric-Furlan, S.; Maraspin, V. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin. Infect. Dis. 2006, 43, 704–710. [Google Scholar] [CrossRef]
- Maraspin, V.; Ruzic-Sabljic, E.; Strle, F. Lyme borreliosis and Borrelia spielmanii. Emerg. Infect. Dis. 2006, 12, 1177. [Google Scholar] [CrossRef]
- Fingerle, V.; Schulte-Spechtel, U.C.; Ruzic-Sabljic, E.; Leonhard, S.; Hofmann, H.; Weber, K.; Pfister, K.; Strle, F.; Wilske, B. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int. J. Med. Microbiol. 2008, 298, 279–290. [Google Scholar]
- Földvári, G.; Farkas, R.; Lakos, A. Borrelia spielmanii erythema migrans, Hungary. Emerg. Infect. Dis. 2005, 11, 1794–1795. [Google Scholar] [CrossRef]
- Ryffel, K.; Péter, O.; Rutti, B.; Suard, A.; Dayer, E. Scored antibody reactivity determined by immunoblotting shows an association between clinical manifestations and presence of Borrelia burgdorferi sensu stricto, B. garinii, B. afzelii, and B. valaisiana in humans. J. Clin. Microbiol. 1999, 37, 4086–4092. [Google Scholar]
- Wang, G.; van Dam, A.P.; Schwartz, I.; Dankert, J. Molecular typing of Borrelia burgdorferi sensu lato: Taxonomic, epidemiological, and clinical implications. Clin. Microbiol. Rev. 1999, 2, 633–653. [Google Scholar] [CrossRef] [Green Version]
- Pritt, B.S.; Respicio-Kingry, L.B.; Sloan, L.M.; Schriefer, M.E.; Replogle, A.J.; Bjork, J.; Liu, G.; Kingry, L.C.; Mead, P.S.; Neitzel, D.F.; et al. Borrelia mayonii sp. nov a member of the Borrelia burgdorferi sensu lato complex, detected in patients and ticks in the upper midwestern United States. Int. J. Syst. Evol. Microbiol. 2016, 66, 4878–4880. [Google Scholar] [CrossRef] [PubMed]
- Collares-Pereira, M.; Couceiro, S.; Franca, I.; Kurtenbach, K.; Schafer, S.M.; Vitorino, L.; Goncalves, L.; Baptista, S.; Vieira, M.L.; Cunha, C. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 2004, 42, 1316–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golovchenko, M.; Vancová, M.; Clark, K.; Oliver, J.H.; Grubhoffer, L.; Rudenko, N. A divergent spirochete strain isolated from a resident of the southeastern United States was identified by multilocus sequence typing as Borrelia bissettii. Parasites Vectors 2016, 9, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudenko, N.; Golovchenko, M.; Mokrácek, A.; Piskunová, N.; Ruzek, D.; Mallatová, N.; Grubhoffer, L. Detection of Borrelia bissettii in cardiac valve tissue of a patient with endocarditis and aortic valve stenosis in the Czech Republic. J. Clin. Microbiol. 2008, 46, 3540–3543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudenko, N.; Golovchenko, M.; Ruzek, D.; Piskunova, N.; Mallátová, N.; Grubhoffer, L. Molecular detection of Borrelia bissettii DNA in serum samples from patients in the Czech Republic with suspected borreliosis. FEMS Microbiol. Lett. 2009, 292, 274–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowicz, M.; Ladstatter, S.; Schotta, A.M.; Reiter, M.; Pomberger, G.; Stanek, G. Oligoarthritis caused by Borrelia bavariensis, Austria, 2014. Emerg. Infect. Dis. 2015, 21, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Tijsse-Klasen, E.; Pandak, N.; Hengeveld, P.; Takumi, K.; Koopmans, M.P.; Sprong, H. Ability to cause erythema migrans differs between Borrelia burgdorferi sensu lato isolates. Parasites Vectors 2013, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.L.; Leydet, B.; Hartman, S. Lyme borreliosis in human patients in Florida and Georgia, USA. Int. J. Med. Sci. 2013, 10, 915–931. [Google Scholar] [CrossRef] [Green Version]
- Rudenko, N.; Goloychenko, M.; Grubhoffer, L.; Oliver, J.H., Jr. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Rollend, L.; Fikrig, E.; Lepore, T.; Barbour, A.; Fish, D. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013, 368, 291. [Google Scholar] [CrossRef] [Green Version]
- Daniel, M.; Rudenko, N.; Golovchenko, M.; Danielová, V.; Fialová, A.; Kříž, B.; Malý, M. The occurrence of Ixodes ricinus ticks and important tick-borne pathogens in areas with high tick-borne encephalitis prevalence in different altitudinal levels of the Czech Republic Part II. Ixodes ricinus ticks and genospecies of Borrelia burgdorferi sensu lato complex. Epidemiol. Mikrobiol. Imunol. 2016, 65, 182–192. [Google Scholar] [PubMed]
- Postic, D.; Garnier, M.; Baranton, G. Multilocus sequence analysis of atypical Borrelia burgdorferi sensu lato isolates-description of Borrelia californiensis sp. nov and genomospecies 1 and 2. Int. J. Med. Microbiol. 2007, 297, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.; Ott-Conn, C.; Worth, J.; Poulsen, A.; Clifford, D. An Ixodes minor and Borrelia carolinensis enzootic cycle involving a critically endangered Mojave Desert rodent. Ecol. Evol. 2014, 4, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Margos, G.; Hojgaard, A.; Lane, R.S.; Cornet, M.; Fingerle, V.; Rudenko, N.; Ogden, N.; Aanensen, D.M.; Fish, D.; Piesman, J. Multilocus sequence analysis of Borrelia bissettii strains from North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks Tick Borne Dis. 2010, 1, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margos, G.; Fedorova, N.; Kleinjan, J.E.; Hartberger, C.; Schwan, T.G.; Sing, A.; Fingerle, V. Borrelia lanei sp. nov. extends the diversity of Borrelia species in California. Int. J. Syst. Evol. Microbiol. 2017, 67, 3872–3876. [Google Scholar] [CrossRef] [Green Version]
- Caulfield, A.J.; Pritt, B.S. Lyme Disease Coinfections in the United States. Clin. Lab. Med. 2015, 35, 827–846. [Google Scholar] [CrossRef]
- Preac Mursic, V.; Marget, W.; Busch, U.; Pleterski Rigler, D.; Hagl, S. Kill kinetics of Borrelia burgdorferi and bacterial findings in relation to the treatment of Lyme borreliosis. Infection 1996, 24, 9–16. [Google Scholar] [CrossRef]
- Stanek, G.; Reiter, M. The expanding Lyme Borrelia complex- clinical significance of genomic species? Clin. Microbiol. Infect. 2011, 17, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Carpi, G.; Kitchen, A.; Kim, H.L.; Ratan, A.; Drautz-Moses, D.I.; McGraw, J.J.; Kazimirova, M.; Rizzoli, A.; Schuster, S.C. Mitogenomes reveal diversity of the European Lyme borreliosis vector Ixodes ricinus in Italy. Mol. Phylogenet Evol. 2016, 101, 194–202. [Google Scholar] [CrossRef]
- Korenberg, E.I.; Nefedova, V.V.; Romanenko, V.N.; Gorelova, N.B. The tick Ixodes pavlovskyi as a host of spirochetes pathogenic for humans and its possible role in the epizootiology and epidemiology of borrelioses. Vector Borne Zoonotic Dis. 2010, 10, 453–458. [Google Scholar] [CrossRef]
- Shpynov, S. Ixodes persulcatus, a major vector of Alphaproteobacteria in Russia. Ticks Tick Borne Dis. 2012, 3, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Taylor, R.C.; Taylor, G.C.; Bosler, E.M. Lyme disease: A proposed ecological index to assess areas of risk in the northeastern United States. Am. J. Public. Health. 1991, 81, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Steinlein, D.B.; Mun, J. Human Behaviors elevating exposure to Ixodes pacificus (Acari: Ixodidae) nymphs and their associated bacterial zoonotic agents in a hardwood forest. J. Med. Entomol. 2004, 41, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubska, L.; Literak, I.; Kocianova, E.; Taragelova, V.; Sychra, O. Differential role of passerine birds in distribution of Borrelia spirochetes, based on data from ticks collected from birds during the postbreeding migration period in Central Europe. Appl. Environ. Microbiol. 2009, 75, 596–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubálek, Z. An annotated checklist of pathogenic microorganisms associated with migratory birds. J. Wild. Dis. 2004, 40, 639–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.D.; Anderson, J.F.; Durden, L.A. Widespread Dispersal of Borrelia burgdorferi –infected ticks collected from songbirds across Canada. J. Parasitol. 2012, 98, 49–59. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, E.; Thomas, G.; Jaenson, T. Lyme Borreliosis in Europe: Influences of Climate and Climate Change, Epidemiology, Ecology and Adaptation Measures. World Health Organization Publication. Available online: http://www.euro.who.int/en/publications/abstracts/lyme-borreliosis-in-europe.-influences-of-climate-and-climate-change,-epidemiology,-ecology-and-adaptation-measures/ (accessed on 30 May 2019).
- Coyle, P.K.; Schutzer, S.E.; Deng, Z.; Krupp, L.B.; Belman, A.L.; Benach, J.L.; Luft, B.J. Detection of Borrelia burgdorferi-specific antigen in antibody-negative cerebrospinal fluid in neurologic Lyme disease. Neurology 1995, 45, 2010–2015. [Google Scholar] [CrossRef]
- Mikkila, H.O.; Seppala, I.J.; Viljanen, M.K.; Peltomaa, M.P.; Karma, A. The expanding clinical spectrum of ocular Lyme borreliosis. Ophthalmology 2000, 107, 581–587. [Google Scholar] [CrossRef]
- Nocton, J.J.; Dressler, F.; Rutledge, B.J.; Rys, P.N.; Persing, D.H.; Steere, A.C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 1994, 330, 229–234. [Google Scholar] [CrossRef]
- Nocton, J.J.; Bloom, B.J.; Rutledge, B.J.; Persing, D.H.; Logigian, E.L.; Schmid, C.H.; Steere, A.C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in cerebrospinal fluid in Lyme neuroborreliosis. J. Infect. Dis. 1996, 174, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Kalimo, H.; Marttila, R.J.; Marjamäki, M.; Sonninen, P.; Nikoskelainen, J.; Viljanen, M.K. Inflammatory brain changes in Lyme borreliosis. A report on three patients and review of literature. Brain 1996, 119, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Preac-Mursic, V.; Pfister, H.W.; Spiegel, H.; Burk, R.; Wilske, B.; Reinhardt, S.; Böhmer, R. First isolation of Borrelia burgdorferi from an iris biopsy. J. Clin. Neuro Ophthalmol. 1993, 13, 155–161. [Google Scholar]
- Aberer, E.; Kersten, A.; Klade, H.; Poitschek, C.; Jurecka, W. Heterogeneity of Borrelia burgdorferi in the skin. Am. J. Dermatopathol. 1996, 18, 571–579. [Google Scholar] [CrossRef]
- Chmielewski, T.; Tylewska-Wierzhanowska, S. Inhibition of fibroblast apoptosis by Borrelia afzelii, Coxiella burnetii and Bartonella henselae. Pol. J. Microbiol. 2011, 60, 269–272. [Google Scholar] [CrossRef]
- De Koning, J.; Hoogenkamp-Korstanje, J.A.; van der Linde, M.R.; Crijns, H.J. Demonstration of spirochetes in cardiac biopsies of patients with Lyme disease. J. Infect. Dis. 1989, 160, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Dorward, D.W.; Fischer, E.R.; Brooks, D.M. Invasion and cytopathic killing of human lymphocytes by spirochetes causing Lyme disease. Clin. Infect. Dis. 1991, 25, S2–S8. [Google Scholar] [CrossRef] [Green Version]
- Georgilis, K.; Peacocke, M.; Klempner, M.S. Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro. J. Infect. Dis. 1992, 166, 440–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girschick, H.J.; Huppertz, H.I.; Rüssmann, H.; Krenn, V.; Karch, H. Intracellular persistence of Borrelia burgdorferi in human synovial cells. Rheumatol. Int. 1996, 16, 125–132. [Google Scholar] [CrossRef]
- Häupl, T.; Hahn, G.; Rittig, M.; Krause, A.; Schoerner, C.; Schonherr, U.; Kalden, J.R.; Burmester, G.R. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 1993, 36, 1621–1626. [Google Scholar] [CrossRef]
- Klempner, M.S.; Noring, R.; Rogers, R.A. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J. Infect. Dis. 1993, 167, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Livengood, J.A.; Gilmore, R.D. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006, 8, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Sturrock, A.; Weis, J.J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect. Immun. 1991, 59, 671–678. [Google Scholar] [PubMed]
- Nanagara, R.; Duray, P.H.; Schumacher, H.R. Ultrastructural demonstration of spirochetal antigens in synovial fluid and synovial membrane in chronic Lyme disease: Possible factors contributing to persistence of organisms. Hum. Pathol. 1996, 27, 1025–1034. [Google Scholar] [CrossRef]
- Stanek, G.; Klein, J.; Bittner, R.; Glogar, D. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N. Engl. J. Med. 1990, 322, 249–252. [Google Scholar] [CrossRef]
- Valesova, M.; Trnavský, K.; Hulínská, D.; Alusík, S.; Janousek, J.; Jirous, J. Detection of Borrelia in the synovial tissue from a patient with Lyme borreliosis by electron microscopy. J. Rheumatol. 1989, 16, 1502–1505. [Google Scholar]
- Duray, P.H.; Steere, A.C. Clinical pathologic correlations of Lyme disease by stage. Ann. N. Y. Acad. Sci. 1988, 539, 65–79. [Google Scholar] [CrossRef]
- Halperin, J.J.; Volkman, D.; Wu, P. Central nervous system abnormalities in Lyme neuroborreliosis. Neurology 1991, 41, 1571–1582. [Google Scholar] [CrossRef]
- Halperin, J.J.; Little, B.W.; Coyle, P.K.; Dattwyler, R.J. Lyme disease: Cause of a treatable peripheral neuropathy. Neurology 1987, 37, 1700–1706. [Google Scholar] [CrossRef]
- Broderick, J.P.; Sandok, B.A.; Mertz, L.E. Focal encephalitis in a young woman 6 years after the onset of Lyme disease: Tertiary Lyme disease? Mayo Clin. Proc. 1987, 62, 313–316. [Google Scholar] [CrossRef] [Green Version]
- Coyle, P.K. Neurologic aspects of Lyme disease. Med. Clin. N. Am. 2002, 86, 261–284. [Google Scholar] [CrossRef]
- Fallon, B.A.; Schwartzberg, M.; Bransfield, R.; Zimmerman, B.; Scotti, A.; Weber, C.A.; Liebowitz, M.R. Late-Stage Neuropsychiatric Lyme Borreliosis: Differential Diagnosis and Treatment. Psychosomatics 1995, 36, 295–300. [Google Scholar] [CrossRef]
- Fallon, B.A.; Nields, J.A. Lyme Disease: A Neuropsychiatric Illness. Am. J. Psychiatry 1994, 151, 1571–1583. [Google Scholar] [PubMed]
- Reik, L.; Steere, A.C.; Bartenhagen, N.H.; Shope, R.E.; Malawista, S.E. Neurologic abnormalities of Lyme disease. Medicine 1979, 58, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Wormser, G.P.; Dattwyler, R.J.; Shapiro, E.D.; Halperin, J.J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, K.; Karabin, B.; Kanjwal, Y.; Grubb, B.P. Postural orthostatic tachycardia syndrome following Lyme disease. Cardiol. J. 2011, 18, 63–66. [Google Scholar] [PubMed]
- Dinerman, H.; Steere, A.C. Lyme disease associated with fibromyalgia. Ann. Intern. Med. 1992, 117, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Bransfield, R.C. The psychoimmunology of lyme/tick-borne diseases and its association with neuropsychiatric symptoms. Open Neurol. J. 2012, 6, 88–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolson, G.L.; Settineri, R.; Ellithorpe, R. Lipid Replacement Therapy with a Glycophospholipid Formulation with NADH and CoQ10 Significantly Reduces Fatigue in Intractable Chronic Fatiguing Illnesses and Chronic Lyme Disease Patients. Int. J. Clin. Med. 2012, 3, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Jutras, B.L.; Savage, C.R.; Arnold, W.K.; Lethbridge, K.G.; Carroll, D.W.; Tilly, K.; Bestor, A.; Zhu, H.; Seshu, J.; Zückert, W.R.; et al. Borrelia burgdorferi peptidoglycan is a persistent antigen in patients with Lyme arthritis. Proc. Natl. Acad. Sci. USA 2019, 116, 13498–13507. [Google Scholar] [CrossRef] [Green Version]
- Bockenstedt, L.K.; Gonzalez, D.G.; Haberman, A.M.; Belperron, A.A. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. J. Clin. Investig. 2012, 122, 2652–2660. [Google Scholar] [CrossRef]
- Arvikar, S.L.; Crowley, J.T.; Sulka, K.B.; Steere, A.C. Autoimmune Arthritides, Rheumatoid Arthritis, Psoriatic Arthritis, or Peripheral Spondyloarthritis Following Lyme Disease. Arthritis Rheumatol. 2017, 69, 194–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccallini, P.; Bonin, S.; Trevisan, G. Autoimmunity against a glycolytic enzyme as a possible cause for persistent symptoms in Lyme disease. Med. Hypotheses 2018, 110, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Levin, E.S.; Schweitzer, P.J.; Hardesty, D. Inflammation and central nervous system Lyme disease. Neurobiol. Dis. 2010, 37, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Strle, K.; Sulka, K.B.; Pianta, A.; Crowley, J.T.; Arvikar, S.L.; Anselmo, A.; Sadreyev, R.; Steere, A.C. T-Helper 17 Cell Cytokine Responses in Lyme Disease Correlate with Borrelia burgdorferi Antibodies During Early Infection and With Autoantibodies Late in the Illness in Patients With Antibiotic-Refractory Lyme Arthritis. Clin. Infect. Dis. 2017, 64, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donta, S.T. Issues in the diagnosis and treatment of lyme disease. Open Neurol. J. 2012, 6, 140–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delong, A.K.; Blossom, B.; Maloney, E.L.; Phillips, S.E. Antibiotic retreatment of Lyme disease in patients with persistent symptoms: A biostatistical review of randomized, placebo-controlled, clinical trials. Contemp. Clin. Trials 2012, 33, 1132–1142. [Google Scholar] [CrossRef]
- Schmidli, J.; Hunziker, T.; Moesli, P.; Schaad, U.B. Cultivation of Borrelia burgdorferi from joint fluid three months after treatment of facial palsy due to Lyme borreliosis. J. Infect. Dis. 1988, 158, 905–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirsch, M.; Ruben, F.L.; Steere, A.C.; Duray, P.H.; Norden, C.W.; Winkelstein, A. Fatal adult respiratory distress syndrome in a patient with Lyme disease. JAMA 1988, 259, 2737–2739. [Google Scholar] [CrossRef] [PubMed]
- Preac-Mursic, V.; Weber, K.; Pfister, H.W.; Wilske, B.; Gross, B.; Baumann, A.; Prokop, J. Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis. Infection 1989, 17, 355–359. [Google Scholar] [CrossRef]
- Pfister, H.W.; Preac-Mursic, V.; Wilske, B.; Schielke, E.; Sorgel, F.; Einhaupl, K.M. Randomized comparison of ceftriaxone and cefotaxime in Lyme neuroborreliosis. J. Infect. Dis. 1991, 163, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Liegner, K.B.; Shapiro, J.R.; Ramsay, D.; Halperin, A.; Hogrefe, W.; Kong, L. Recurrent erythema migrans despite extended antibiotic treatment with minocycline in a patient with persisting Borrelia burgdorferi infection. J. Am. Acad. Dermatol. 1993, 28, 312–314. [Google Scholar] [CrossRef]
- Strle, F.; Preac-Mursic, V.; Cimperman, J.; Ruzic, E.; Maraspin, V.; Jereb, M. Azithromycin versus doxycycline for treatment of erythema migrans: Clinical and microbiological findings. Infection 1993, 21, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.; Wilske, B.; Preac-Mursic, V.; Thurmayr, R. Azithromycin versus penicillin V for the treatment of early Lyme borreliosis. Infection 1993, 21, 367–372. [Google Scholar] [CrossRef]
- Battafarano, D.F.; Combs, J.A.; Enzenauer, R.J.; Fitzpatrick, J.E. Chronic septic arthritis caused by Borrelia burgdorferi. Clin. Orthop. Relat. Res. 1993, 297, 238–241. [Google Scholar]
- Chancellor, M.B.; McGinnis, D.E.; Shenot, P.J.; Kiilholma, P.; Hirsch, I.H. Urinary dysfunction in Lyme disease. J. Urol. 1993, 149, 26–30. [Google Scholar] [CrossRef]
- Bradley, J.F.; Johnson, R.C.; Goodman, J.L. The persistence of spirochetal nucleic acids in active Lyme arthritis. Ann. Intern. Med. 1994, 120, 487–489. [Google Scholar] [CrossRef]
- Lawrence, C.; Lipton, R.B.; Lowy, F.D.; Coyle, P.K. Seronegative chronic relapsing neuroborreliosis. Eur. Neurol. 1995, 35, 113–117. [Google Scholar] [CrossRef]
- Strle, F.; Maraspin, V.; Lotric-Furlan, S.; Ruzic-Sabljic, E.; Cimperman, J. Azithromycin and doxycycline for treatment of Borrelia culture-positive erythema migrans. Infection 1996, 24, 64–68. [Google Scholar] [CrossRef]
- Oksi, J.; Nikoskelainen, J.; Viljanen, M.K. Comparison of oral cefixime and intravenous ceftriaxone followed by oral amoxicillin in disseminated Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 715–719. [Google Scholar] [CrossRef]
- Priem, S.; Burmester, G.R.; Kamradt, T.; Wolbart, K.; Rittig, M.G.; Krause, A. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann. Rheum. Dis. 1998, 57, 118–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, B.J.; Stewart, M.; Lennox, V.A.; Fukunaga, M.; Yabuki, M.; Macorison, H.; Kitchener-Smith, J. Culture-positive Lyme borreliosis. Med. J. Aust. 1998, 168, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Oksi, J.; Marjamaki, M.; Nikoskelainen, J.; Viljanen, M.K. Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis. Ann. Med. 1999, 31, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Breier, F.; Khanakah, G.; Stanek, G.; Kunz, G.; Aberer, E.; Schmidt, B.; Tappeiner, G. Isolation and polymerase chain reaction typing of Borrelia afzelii from a skin lesion in a seronegative patient with generalized ulcerating bullous lichen sclerosus et atrophicus. Br. J. Dermatol. 2001, 144, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Hunfeld, K.P.; Ruzic-Sabljic, E.; Norris, D.E.; Kraiczy, P.; Strle, F. In vitro susceptibility testing of Borrelia burgdorferi sensu lato isolates cultured from patients with erythema migrans before and after antimicrobial chemotherapy. Antimicrob. Agents Chemother. 2005, 49, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Marques, A.; Telford, S.R.; Turk, S.P.; Chung, E.; Williams, C.; Dardick, K.; Krause, P.J.; Brandeburg, C.; Crowder, C.D.; Carolan, H.E.; et al. Xenodiagnosis to detect Borrelia burgdorferi infection: A first-in-human study. Clin. Infect. Dis. 2014, 58, 937–945. [Google Scholar] [CrossRef]
- Embers, M.E.; Barthold, S.W.; Borda, J.T.; Bowers, L.; Doyle, L.; Hodzic, E.; Jacobs, M.B.; Hasenkampf, N.R.; Martin, D.S.; Narasimhan, S.; et al. Persistence of Borrelia burgdorferi in Rhesus Macaques following antibiotic treatment of disseminated infection. PLoS ONE 2012, 7, e29914. [Google Scholar] [CrossRef]
- Hodzic, E.; Feng, S.; Holden, K.; Freet, K.J.; Barthold, S.W. Persistence of Borrelia burgdorferi following antibiotic treatment in mice. Antimicrob. Agents Chemother. 2008, 52, 1728–1736. [Google Scholar] [CrossRef] [Green Version]
- Barthold, S.W.; Hodzic, E.; Imai, D.M.; Feng, S.; Yang, X.; Luft, B.L. Ineffectiveness of tigecycline against persistent Borrelia burgdorferi. Antimicrob. Agents Chemother. 2010, 54, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Straubinger, R.K.; Summers, B.A.; Chang, Y.F.; Appel, M.J. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 1997, 35, 111–116. [Google Scholar]
- Embers, M.E.; Hasenkampf, N.R.; Jacobs, M.B.; Tardo, A.C.; Doyle-Meyers, L.A.; Philipp, M.T.; Hodzic, E. Variable manifestations, diverse seroreactivity and post-treatment persistence in non-human primates exposed to Borrelia burgdorferi by tick feeding. PLoS ONE 2017, 12, e0189071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudenko, N.; Golovchenko, M.; Kybicova, K.; Vancova, M. Metamorphoses of Lyme disease spirochetes: Phenomenon of Borrelia persisters. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tager, F.A.; Fallon, B.A.; Keilp, J.; Rissenberg, M.; Jones, C.R.; Liebowitz, M.R. A Controlled Study of Cognitive Deficits in Children with Chronic Lyme Disease. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.W.; Preac-Mursic, V.; Wilske, B.; Einhäupl, K.M.; Weinberger, K. Latent Lyme neuroborreliosis: Presence of Borrelia burgdorferi in the cerebrospinal fluid without concurrent inflammatory signs. Neurology 1989, 39, 1118. [Google Scholar] [CrossRef]
- Oksi, J.; Nikoskelainen, J.; Hiekkanen, H.; Lauhio, A.; Peltomaa, M.; Pitkäranta, A.; Nyman, D.; Granlund, H.; Carlsson, S.A.; Seppälä, I.; et al. Duration of antibiotic treatment in disseminated Lyme borreliosis: A double-blind, randomized, placebo-controlled, multicenter clinical study. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 571–581. [Google Scholar] [CrossRef]
- Fraser, D.D.; Kong, L.I.; Miller, F.W. Molecular detection of persistent Borrelia burgdorferi in a man with dermatomyositis. Clin. Exp. Rheumatol. 1992, 10, 387–390. [Google Scholar]
- Frey, M.; Jaulhac, B.; Piemont, Y.; Marcellin, L.; Boohs, P.M.; Vautravers, P.; Jessel, M.; Kuntz, J.L.; Monteil, H.; Sibilia, J. Detection of Borrelia burgdorferi DNA in muscle of patients with chronic myalgia related to Lyme disease. Am. J. Med. 1998, 104, 591–594. [Google Scholar] [CrossRef]
- Brouqui, P.; Badiaga, S.; Raoult, D. Eucaryotic cells protect Borrelia burgdorferi from the action of penicillin and ceftriaxone but not from the action of doxycycline and erythromycin. Antimicrob. Agents Chemother. 1996, 40, 1552–1554. [Google Scholar] [CrossRef] [Green Version]
- Hodzic, E.; Feng, S.; Freet, K.J.; Barthold, S.W. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect. Immun. 2003, 71, 5042–5055. [Google Scholar] [CrossRef] [Green Version]
- Embers, M.E.; Ramamoorthy, R.; Philipp, M.T. Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease. Microbes Infect. 2004, 6, 312–318. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Newman, S.A. Hidden in plain sight: Borrelia burgdorferi and the extracellular matrix. Trends Microbiol. 2007, 15, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Szczepanski, A.; Benach, J.L. Lyme borreliosis: Host responses to Borrelia burgdorferi. Microbiol. Mol. Biol. Rev. 1991, 55, 21–34. [Google Scholar]
- Sapi, E.; Bastian, S.L.; Mpoy, C.M.; Scott, S.; Rattelle, A.; Pabbati, N.; Poruri, A.; Burugu, D.; Theophilus, P.A.; Pham, T.V.; et al. Characterization of biofilm formation byBorrelia burgdorferi in vitro. PLoS ONE 2012, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sapi, E.; Balasubramanian, K.; Poruri, A.; Maghsoudlou, J.S.; Socarras, K.M.; Timmaraju, A.V.; Filush, K.R.; Gupta, K.; Shaikh, S.; Theophilus, P.A.; et al. Evidence of in vivo existence of Borrelia biofilm in Borrelial lymphocytomas. Eur. J. Microbiol. Immunol. 2016, 6, 9–24. [Google Scholar] [CrossRef] [Green Version]
- Sapi, E.; Kasliwala, R.S.; Ismail, H.; Torres, J.P.; Oldakowski, M.; Markland, S.; Gaur, G.; Melillo, A.; Eisendle, K.; Liegner, K.B.; et al. The Long-Term Persistence of Borrelia burgdorferi Antigens and DNA in the Tissues of a Patient with Lyme Disease. Antibiotics 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.R.; Hardham, J.M.; Barbour, A.G.; Norris, S.J. Antigenic Variation in Lyme Disease Borreliae by Promiscuous Recombination of VMP-like Sequence Cassettes. Cell 1997, 89, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Coutte, L.; Botkin, D.J.; Gao, L.; Norris, S.J. Detailed Analysis of Sequence Changes Occurring during vlsE Antigenic Variation in the Mouse Model of Borrelia burgdorferi Infection. PLoS Pathog. 2009, 5, e1000293. [Google Scholar] [CrossRef]
- Liang, F.T.; Jacobs, M.B.; Bowers, L.C.; Philipp, M.T. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J. Exp. Med. 2002, 195, 415–422. [Google Scholar] [CrossRef]
- Barbour, A.G.; Restrepo, B.I. Antigenic variation in vector-borne pathogens. Emerg. Infect. Dis. 2000, 6, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Schwan, T.G.; Piesman, J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 2000, 38, 382–388. [Google Scholar]
- Mursic, V.P.; Wanner, G.; Reinhardt, S.; Wilske, B.; Busch, U.; Marget, W. Formation and cultivation of Borrelia spheroplast variants. Infection 1996, 24, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Al-Robaiy, S.; Dihazi, H.; Kacza, J.; Seeger, J.; Schiller, J.; Huster, D.; Knauer, J.; Straubinger, R.K. Metamorphosis of Borrelia burgdorferi organisms–RNA, lipid and protein composition in context with the spirochetes’ shape. J. Basic Microbiol. 2010, 50, S5–S17. [Google Scholar] [CrossRef] [PubMed]
- Duray, P.H.; Yin, S.R.; Berzukov, L.; Cox, C.; Cho, M.S.; Fitzgerald, W.; Dorward, D.; Zimmerberg, J.; Margolis, L. Invasion of human tissue ex vivo by Borrelia burgdorferi. J. Infect. Dis. 2005, 191, 1747–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersten, A.; Poitschek, C.; Rauch, S.; Aberer, E. Effects of penicillin, ceftriaxone, and doxycycline on morphology of Borrelia burgdorferi. Antimicrob. Agents Chemother. 1995, 39, 1127–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alban, P.S.; Johnson, P.W.; Nelson, D.R. Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi. Microbiology 2000, 146, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Brorson, O.; Brorson, S.H. In vitro conversion of Borrelia burgdorferi to cystic forms in spinal fluid, and transformation to mobile spirochetes by incubation in BSK-H medium. Infection 1998, 26, 144–150. [Google Scholar] [CrossRef]
- Kraiczy, P.; Hellwage, J.; Skerka, C.; Becker, H.; Kirschfink, M.; Simon, M.M.; Brade, V.; Zipfel, P.F.; Wallich, R. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J. Biol. Chem. 2004, 279, 2421–2429. [Google Scholar] [CrossRef] [Green Version]
- Pausa, M.; Pellis, V.; Cinco, M.; Giulianini, P.G.; Presani, G.; Perticarari, S.; Murgia, R.; Tedesco, F. Serum-resistant strains of Borrelia burgdorferi evade complement-mediated killing by expressing a CD59-like complement inhibitory molecule. J. Immunol. 2003, 170, 3214–3222. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhi, H.; Garrigues, R.J.; Keightley, A.; Garcia, B.L.; Skare, J.T. Structural determination of the complement inhibitory domain of Borrelia burgdorferi BBK32 provides insight into classical pathway complement evasion by Lyme disease spirochetes. PLoS Pathog. 2019, 15, e1007659. [Google Scholar] [CrossRef] [Green Version]
- Hartiala, P.; Hytönen, J.; Suhonen, J.; Leppäranta, O.; Tuominen-Gustafsson, H.; Viljanen, M.K. Borrelia burgdorferi inhibits human neutrophil functions. Microbes Infect. 2008, 10, 60–68. [Google Scholar] [CrossRef]
- Hartiala, P.; Hytönen, J.; Pelkonen, J.; Kimppa, K.; West, A.; Penttinen, M.A.; Suhonen, J.; Lahesmaa, R.; Viljanen, M.K. Transcriptional response of human dendritic cells to Borrelia Garinii-defective CD38 and CCR7 expression detected. J. Leukoc. Biol. 2007, 82, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Tunev, S.S.; Hastey, C.J.; Hodzic, E.; Feng, S.L.; Barthold, S.W.; Baumgarth, N. Lymphoadenopathy during lyme borreliosis is caused by spirochete migration-induced specific B cell activation. PLoS Pathog. 2011, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastey, C.J.; Elsner, R.A.; Barthold, S.W.; Baumgarth, N. delays and diversions mark the development of B cell responses to Borrelia burgdorferi infection. J. Immunol. 2012, 188, 5612–5622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsner, R.A.; Hastey, C.J.; Baumgarth, N. CD4 (+) T cells promote antibody production but not sustained affinity maturation during Borrelia burgdorferi infection. Infect. Immun. 2015, 83, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Lazarus, J.J.; Kay, M.A.; McCarter, A.L.; Wooten, R.M. Viable Borrelia burgdorferi enhances interleukin-10 production and suppresses activation of murine macrophages. Infect. Immun. 2008, 76, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Giambartolomei, G.H.; Dennis, V.A.; Philipp, M.T. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect. Immun. 1998, 66, 2691–2697. [Google Scholar]
- Feng, J.; Wang, T.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. Identification of novel activity against Borrelia burgdorferi persisters using an FDA approved drug library. Emerg. Microbes Infect. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Feng, J.; Auwaerter, P.G.; Zhang, Y. Drug combinations against Borrelia burgdorferi persisters in vitro: Eradication achieved by using daptomycin, cefoperazone and doxycycline. PLoS ONE 2015, 10, e0117207. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Shi, W.; Zhang, S.; Sullivan, D.; Auwaerter, P.G.; Zhang, Y. A drug combination screen identifies drugs active against amoxicillin-induced round bodies of in vitro Borrelia burgdorferi persisters from an FDA drug library. Front. Microbiol. 2016, 7, 743. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Brown, A.V.; Matluck, N.E.; Hu, L.T.; Lewis, K. Borrelia burgdorferi, the Causative Agent of Lyme Disease, Forms Drug-Tolerant Persister Cells. Antimicrob. Agents Chemother. 2015, 59, 4616–4624. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Li, T.; Yee, R.; Yuan, Y.; Bai, C.; Cai, M.; Shi, W.; Embers, M.; Brayton, C.; Saeki, H.; et al. Stationary phase persister/biofilm microcolony of Borrelia burgdorferi causes more severe disease in a mouse model of Lyme arthritis: Implications for understanding persistence, Post-treatment Lyme Disease Syndrome (PTLDS), and treatment failure. Discov. Med. 2019, 27, 125–138. [Google Scholar] [PubMed]
- Steere, A.C. Lyme disease. N. Engl. J. Med. 1989, 321, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; Malawista, S.E.; Newman, J.H.; Spieler, P.N.; Bartenhagen, N.H. Antibiotic therapy in Lyme disease. Ann. Intern. Med. 1980, 93, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Citera, M.; Freeman, P.R.; Horowitz, R.I. Empirical validation of the Horowitz Multiple Systemic Infectious Disease Syndrome Questionnaire for suspected Lyme disease. Int. J. Gen. Med. 2017, 10, 249–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norris, S.J. Antigenic variation with a twist–the Borrelia story. Mol. Microbiol. 2006, 60, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Lantos, P.M. Chronic Lyme disease. Infect. Dis. Clin. N. Am. 2015, 29, 325–340. [Google Scholar] [CrossRef]
- Fallon, B.A.; Petkova, E.; Keilp, J.G.; Britton, C.B. A reappraisal of the U.S. Clinical trials of post-treatment lyme disease syndrome. Open Neurol. J. 2012, 6, 79–87. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Lyme Disease: Diagnosis and Management [L] Evidence Review for the Management of Ongoing Symptoms Related to Lyme Disease. NICE Guideline 95 Evidence Review April 2018. Available online: https://www.nice.org.uk/guidance/ng95/evidence/l-management-of-ongoing-symptoms-related-to-lyme-disease-pdf-172521756184 (accessed on 4 August 2019).
- Brzonova, I.; Wollenberg, A.; Prinz, J.C. Acrodermatitis chronica atrophicans affecting all four limbs in an 11-year-old girl. Br. J. Dermatol. 2002, 147, 375–378. [Google Scholar] [CrossRef]
- Müller, D.E.; Itin, P.H.; Büchner, S.A.; Rufli, T. Acrodermatitis chronica atrophicans involving the face. Evidence for Borrelia burgdorferi infection confirmed by DNA amplification. Dermatology 1994, 189, 430–431. [Google Scholar] [CrossRef]
- Haddad, O.; Gillinov, M.; Fraser, T.; Shrestha, N.; Pettersson, G.B. Mitral Valve Endocarditis: A rare Manifestation of Lyme Disease. Ann. Thorac. Surg. 2019, 108, e85–e86. [Google Scholar] [CrossRef]
- Kempf, W.; Kazakov, D.V.; Hübscher, E.; Gugerli, O.; Gerbig, A.W.; Schmid, R.; Palmedo, G.; Kutzner, H. Cutaneous borreliosis associated with T cell-predominant infiltrates: A diagnostic challenge. J. Am. Acad. Dermatol. 2015, 72, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Leslie, T.A.; Levell, N.J.; Cutler, S.J.; Cann, K.J.; Smith, M.E.; Wright, D.J.; Gilkes, J.J.; Robinson, T.W. Acrodermatitis chronica atrophicans: A case report and review of the literature. Br. J. Dermatol. 1994, 131, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Muellegger, R.R.; Schluepen, E.M.; Millner, M.M.; Soyer, H.P.; Volkenandt, M.; Kerl, H. Acrodermatitis chronica atrophicans in an 11-year-old girl. Br. J. Dermatol. 1996, 135, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Stinco, G.; Trevisan, G.; Martina Patriarca, M.; Ruscio, M.; Di Meo, N.; Patrone, P. Acrodermatitis chronica atrophicans of the face: A case report and a brief review of the literature. Acta Dermatovenerol. Croat. 2014, 22, 205–208. [Google Scholar]
- Da Franca, I.; Santos, L.; Mesquita, T.; Collares-Pereira, M.; Baptista, S.; Vieira, L.; Viana, I.; Vale, E.; Prates, C. Lyme borreliosis in Portugal caused by Borrelia lusitaniae? Clinical report on the first patient with a positive skin isolate. Wien. Klin. Wochenschr. 2005, 117, 429–432. [Google Scholar] [CrossRef]
- Zamponi, N.; Cardinali, C.; Tavoni, M.A.; Porfiri, L.; Rossi, R.; Manca, A. Chronic neuroborreliosis in infancy. Ital. J. Neurol. Sci. 1999, 20, 303–307. [Google Scholar] [CrossRef]
- Karma, A.; Pirttila, T.A.; Viljanen, M.K.; Lahde, Y.E.; Raitta, C.M. Secondary retinitis pigmentosa and cerebral demyelination in Lyme borreliosis. Br. J. Ophthalmol. 1993, 77, 120–122. [Google Scholar] [CrossRef] [Green Version]
- Murillo, G.; Ramírez, B.; Romo, L.A.; Muñoz-Sanz, A.; Hileeto, D.; Calonge, M. Oculopalpebral borreliosis as an unusual manifestation of Lyme disease. Cornea 2013, 32, 87–90. [Google Scholar] [CrossRef]
- Oksi, J.; Marjamäki, M.; Koski, K.; Nikoskelainen, J.; Viljanen, M.K. Bilateral facial palsy and meningitis caused by Borrelia double infection. Lancet 1995, 345, 1583–1584. [Google Scholar] [CrossRef]
- Oksi, J.; Kalimo, H.; Marttila, R.J.; Marjamäki, M.; Sonninen, P.; Nikoskelainen, J.; Viljanen, M.K. Intracranial aneurysms in three patients with disseminated Lyme borreliosis: Cause or chance association? J. Neurol. Neurosurg. Psychiatry 1998, 64, 636–642. [Google Scholar] [CrossRef] [Green Version]
- Berger, T.G.; Schoerner, C.; Schell, H.; Simon, M.; Schuler, G.; Röllinghoff, M.; Gessner, A. Two unusual cases of diffuse acrodermatitis chronica atrophicans seronegative for Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 392–395. [Google Scholar] [CrossRef]
- Kaufman, L.D.; Gruber, B.L.; Phillips, M.E.; Benach, J.L. Late cutaneous Lyme disease: Acrodermatitis chronica atrophicans. Am. J. Med. 1989, 86, 828–830. [Google Scholar] [CrossRef]
- Maimone, D.; Villanova, M.; Stanta, G.; Bonin, S.; Malandrini, A.; Guazzi, G.C.; Annunziata, P. Detection of Borrelia burgdorferi DNA and complement membrane attack complex deposits in the sural nerve of a patient with chronic polyneuropathy and tertiary Lyme disease. Muscle Nerve 1997, 20, 969–975. [Google Scholar] [CrossRef]
- Feder, H.M., Jr.; Abeles, M.; Bernstein, M.; Whitaker-Worth, D.; Grant-Kels, J.M. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin. Dermatol. 2006, 24, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Karch, H.; Huppertz, H.I. Repeated detection of Borrelia burgdorferi DNA in synovial fluid of a child with Lyme arthritis. Rheumatol. Int. 1993, 12, 227–229. [Google Scholar] [CrossRef]
- Snydman, D.R.; Schenkein, D.P.; Berardi, V.P.; Lastavica, C.C.; Pariser, K.M. Borrelia burgdorferi in joint fluid in chronic Lyme arthritis. Ann. Intern. Med. 1986, 104, 798–800. [Google Scholar] [CrossRef]
- Chary-Valckenaere, I.; Jaulhac, B.; Champigneulle, J.; Piemont, Y.; Mainard, D.; Pourel, J. Ultrastructural demonstration of intracellular localization of Borrelia burgdorferi in Lyme arthritis. Br. J. Rheumatol. 1998, 37, 468–470. [Google Scholar] [CrossRef]
- Reimers, C.D.; de Koning, J.; Neubert, U.; Preac-Mursic, V.; Koster, J.G.; Müller-Felber, W.; Pongratz, D.E.; Duray, P.H. Borrelia burgdorferi myositis: Report of eight patients. J. Neurol. 1993, 240, 278–283. [Google Scholar] [CrossRef]
- Leverkus, M.; Finner, A.M.; Pokrywka, A.; Franke, I.; Gollnick, H. Metastatic squamous cell carcinoma of the ankle in long-standing untreated acrodermatitis chronica atrophicans. Dermatology 2008, 217, 215–218. [Google Scholar] [CrossRef]
- Miklossy, J.; Khalili, K.; Gern, L.; Ericson, R.L.; Darekar, P.; Bolle, L.; Hurlimann, J.; Paster, B.J. Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J. Alzheimers Dis. 2004, 6, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Rigot, E.; Hantz, V.D.; Labrousse, F.; Martin, C.; Dubos, M.; Assikar, S.; Sparsa, A.; Bonnetblanc, J.M.; Bedane, C. Unusual cutaneous manifestations of late Lyme borreliosis. Eur. J. Dermatol. 2015, 25, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Bauvin, O.; Schmutz, J.L.; De Martino, S.; Busato, T.; Cribier, B.; Barbaud, A.; Wahl, D.; Bursztejn, A.C. A foot tumour as late cutaneous Lyme borreliosis: A new entity? Br. J. Dermatol. 2017, 177, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Morruzzi, C.; Barbarini, A.; Sordet, C.; Cribier, B.; Jaulhac, B.; Lipsker, D. Clinical images: Toe dactylitis revealing late Lyme borreliosis. Arthritis Rheum. 2012, 64, 1293. [Google Scholar] [CrossRef] [PubMed]
- Matera, G.; Labate, A.; Quirino, A.; Lamberti, A.G.; BorzÃ, G.; Barreca, G.S.; Mumoli, L.; Peronace, C.; Giancotti, A.; Gambardella, A.; et al. Chronic neuroborreliosis by B. garinii: An unusual case presenting with epilepsy and multifocal brain MRI lesions. New Microbiol. 2014, 37, 393–397. [Google Scholar]
- Lobraiko, J.; Butler, A.; Petrini, J.; Ahmadi, R. New insights into stages of Lyme disease symptoms from a novel hospital-based registry. J. Prim. Care Community Health 2014, 5, 284–287. [Google Scholar] [CrossRef]
- Rebman, A.W.; Bechtold, K.T.; Yang, T.; Mihm, E.A.; Soloski, M.J.; Novak, C.B.; Aucott, J.N. The Clinical, Symptom, and Quality-of-Life Characterization of a Well-Defined Group of Patients with Posttreatment Lyme Disease Syndrome. Front. Med. 2017, 4, 224. [Google Scholar] [CrossRef] [Green Version]
- Lantos, P.; Rumbaugh, J.; Bockenstedt, L.; Falck-Ytter, Y.T.; Aguero-Rosenfeld, M.E.; Auwaerter, P.G.; Baldwin, K.; Bannuru, R.; Belani, K.K.; Bowie, W.R.; et al. Bowie WRDraft Clinical Practice Guidelines by the Infectious Diseases Society of 1 America (IDSA), American Academy of Neurology (AAN), and 2 American College of Rheumatology (ACR): 2019 Guidelines for the 3 Prevention, Diagnosis and Treatment of Lyme Disease. Available online: https://view.protectedpdf.com/ad6GFZ (accessed on 1 December 2019).
- Eldin, C.; Jaulhac, B.; Mediannikov, O.; Arzouni, J.P.; Raoult, D. Values of diagnostic tests for the various species of spirochetes. Med. Mal. Infect. 2019, 49, 102–111. [Google Scholar] [CrossRef]
- Schutzer, S.E.; Body, B.A.; Boyle, J.; Branson, B.M.; Dattwyler, R.J.; Fikrig, E.; Gerald, N.J.; Gomes-Solecki, M.; Kintrup, M.; Ledizet, M.; et al. Direct Diagnostic Tests for Lyme Disease. Clin. Infect. Dis. 2019, 68, 1052–1057. [Google Scholar] [CrossRef]
| CLD-U: Symptoms, Signs, and Conditions in Patients with Direct Evidence of Infection | ||
|---|---|---|
| Symptoms and Signs | ||
| Constitutional | Skin | Cardiopulmonary |
| Fatigue [71,72,75,106,182,183,184] Fever [75] Weight gain [182] | Atrophic lesions [183] Dry skin [182] Rash, unspecified [185] Skin discoloration [182,183,186,187,188] | Cardiac arrhythmia [138,183,189] Dyspnea [184] Mitral regurgitation [184] Palpitations [184,192] Orthostatic Intolerance [106] |
| Head Ears Eyes Nose Throat (HEENT) | Musculoskeletal | Neuropsychiatric/Neurological |
| Blurred vision [76] Double vision [190] Progressive visual loss [72] Decreased visual acuity [191] Nystagmus [190] Photophobia [192,193] Eyelid swelling [192] Facial flushing [75] Facial pain [72,138] Tinnitus [76] Headache [74,75,76,189,194] Stiff neck [74,75] Hearing loss [55,189] | Arthralgia [106,182,186,195,196,197,198] Arthritis [73,75,124,183,198,199,200,201] Joint swelling [72,106,183,186,198,200] Morning stiffness [196] Muscle cramps [197] Muscle weakness [189,202] Myalgia [106,193,202] Muscle atrophy [197,199,202] | Memory difficulties [74,75,106,194] Abnormal taste [76] Dizziness [75,138] Vertigo [76] Decreased sensation [106,197,201] Paresthesias [189,190,196,201] Tingling [197] Pain, generalized [138,197] Pain radicular [76,191] Decreased dexterity [197] Abnormal gait [75,192,197,199] Abnormal balance [138,191] Limb paralysis [183] Spastic paraparesis [197] Positive Babinski [197] Areflexia [191,201] Hyperreflexia [197] Fasciculations [197] Urinary incontinence [197] Decreased concentration [106] |
| Conditions | ||
| Acrodermatitis chronica atrophicans [76,182,185,186,187,188,195,196,202,203] Alzheimer’s disease [204] Anectoderma [205] Carpal tunnel syndrome [189] Cutaneous tumor [206] Dactylitis [207] | Encephalomyelitis [74,75] Encephalopathy [74,75] Endocarditis [184] Epilepsy/seizure [190,194,208] Facial palsy [74,75,193,208] Meningitis [74,75,193] Mitral regurgitation [184] Mycosis fungoides-like rash [185] | Panuveitis [76] Polyarthritis [202] Radiculoneuropathy [74,75] Sensory-motor polyneuropathy [74,197] Sensory neuropathy [75] Synovitis [189,200] Ulcerative keratitis [192] |
| CLD-PT: Symptoms, Signs, and Conditions in Patients with Direct Evidence of Infection | ||
|---|---|---|
| Symptoms and Signs | ||
| Constitutional | Skin | Cardiopulmonary |
| Anorexia [119] Fatigue [22,71,72,113,119,125,136] Fever [113,137,138] Weight loss [22] | Recurrent EM lesions [23,125] | |
| HEENT | Musculoskeletal | Neuropsychiatric/Neurological |
| Conjunctival irritation [72] Decreased central vision [83] Diplopia [126] Eye pain [72] Photophobia [72] Retro-orbital pain [121] Tinnitus [72] Drooling [22] Fullness in head [125] Headache [71,74,113,126,136,137] Neck pain [22] Stiff neck/torticollis [74] | Arthralgia [23,71,76,83,118,125,126,136,137] Arthritis [73,126] Hand pain [22] Joint swelling [118] Migratory pain [23,126] Muscle stiffness [22] Muscle weakness [139,140] Myalgia [125,126,138,140] Trigger finger [83] | Cognitive dysfunction [119] Poor concentration [125] Memory difficulties [22,74,119,125] Vertigo [121] Dizziness [126] Hypoalgesia [76] Hypoesthesia [76,121] Paresthesias [71,119] Radicular pain [119,137] Cogwheel rigidity [22] Tremors [22] |
| Gastrointestinal | Genitourinary | |
| Vomiting [76] | Nocturia [119] Urge incontinence [22,119] Urgency [119] Urinary frequency [119] | |
| Conditions | ||
| Carpel tunnel syndrome [126] Chorioretinitis [126] Choroiditis [83] Depressed corneal reflexes [121] Encephalitis [126] Encephalomyelitis [74] Encephalomyeloradiculopathy, recurrent [71] | Encephalopathy [74,126] Epilepsy [126] Facial palsy [74] Hepatopathy [126] Hemiparesis [121,126] Meningismus [113] Meningitis [74,126] Mononeuritis multiplex [121] Neuropathy [126] Pericarditis [126] | Pleuritis [126] Radiculitis [126] Radiculoneuropathy [74] Sensory neuropathy [74] Tenosynovitis [83] Trigeminal sensory neuropathy [121] Uveitis [126] Vasculitis [126] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shor, S.; Green, C.; Szantyr, B.; Phillips, S.; Liegner, K.; Burrascano, J.J., Jr.; Bransfield, R.; Maloney, E.L. Chronic Lyme Disease: An Evidence-Based Definition by the ILADS Working Group. Antibiotics 2019, 8, 269. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040269
Shor S, Green C, Szantyr B, Phillips S, Liegner K, Burrascano JJ Jr., Bransfield R, Maloney EL. Chronic Lyme Disease: An Evidence-Based Definition by the ILADS Working Group. Antibiotics. 2019; 8(4):269. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040269
Chicago/Turabian StyleShor, Samuel, Christine Green, Beatrice Szantyr, Steven Phillips, Kenneth Liegner, Joseph Jr. Burrascano, Jr., Robert Bransfield, and Elizabeth L. Maloney. 2019. "Chronic Lyme Disease: An Evidence-Based Definition by the ILADS Working Group" Antibiotics 8, no. 4: 269. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics8040269





