Biosynthesis of Galactan in Mycobacterium tuberculosis as a Viable TB Drug Target?
Abstract
:1. Introduction
2. Structure of the Galactan Component of the Mycobacterial Cell Wall Core: From History to Current Understanding
3. Biosynthesis of Mycobacterial Galactan: Discovery of the Metabolic Pathway
4. UGM, GlfT1, and GlfT2—The Three Key Enzymes with Unexpected Properties
4.1. UDP-Galactopyranose Mutase and the Origin of Mycobacterial Galactofuranose
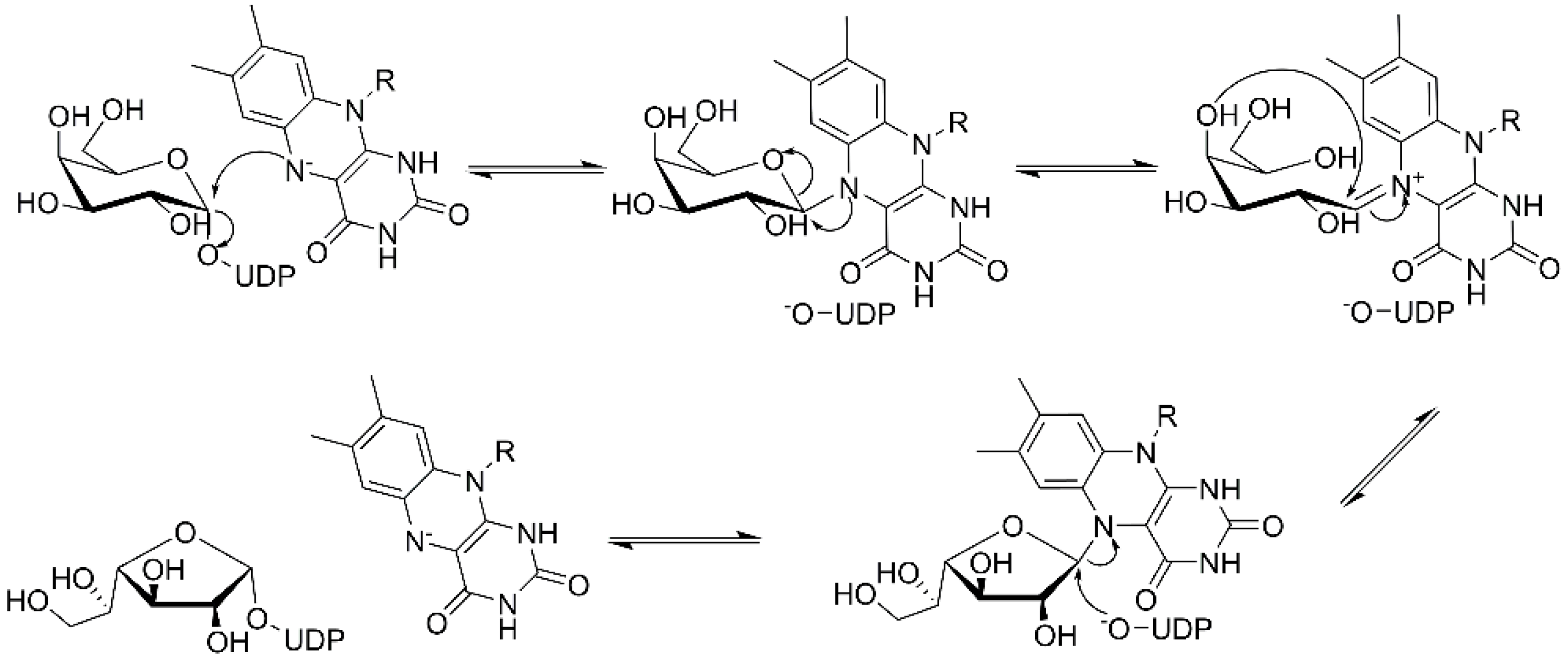
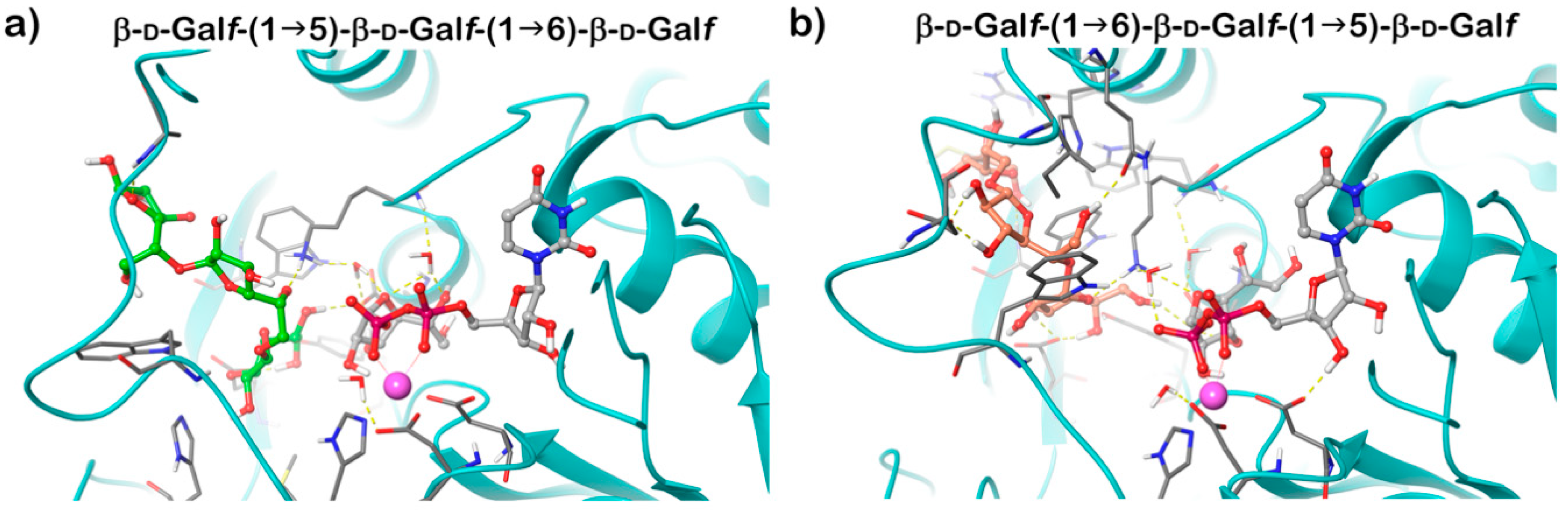
4.2. GlfT2—Processive Bifunctional Galactofuranosyltransferase with One Active Site
4.3. GlfT1—The Enzyme in the Shadow of its More Popular Twin
5. Search for Inhibitors of the Galactan Pathway
5.1. UGM Assays and Inhibitors
| Entry | Year | Compounds Origin | Structure [Reference] | Compound Id 1 | Assay, Inhibitory Activity | Growth Inhibition |
|---|---|---|---|---|---|---|
| 1997 | [79] | HPLC reverse 2 | ||||
| 1 | structure-based |  | (1) | 67% at 200 μg/mL | ||
| 2 | structure-based |  | (2) | 81% at 200 μg/mL | ||
| 2003 | [80] | Formaldehyde release 3 | ||||
| 3 | compound library screening |  | (320KAW73) | IC50 6 μM | No activity on Mtb in vitro | |
| 2004 | [81] | HPLC reverse 3 | MIC | |||
| 4 | compound library screening |  | (1) | IC50 12 μg/mL | 1.6 µg/mL (Mtb H37Ra) | |
| 5 | SAR of (1) |  | (10) | IC50 15 mM | 0.8 µg/mL (Mtb H37Ra) | |
| 6 | SAR of (1) |  | (11) | IC50 11 mM | 1.6 µg/mL (Mtb H37Ra) | |
| 7 | SAR of (1) |  | (23) | IC50 23 mM | 0.2 µg/mL (Mtb H37Ra) | |
| 2006 | [84] | HPLC reverse 4 | ||||
| 8 | designed probe |  | (1) | IC50 5.7 μM | ||
| 9 | compound library screening |  | (6) | IC50 65 μM | ||
| 10 | compound library screening |  | (9) | IC50 41 μM | ||
| 11 | compound library screening |  | (10) | IC50 28 μM | ||
| 2008 | [86] | HPLC reverse 4 | MIC | |||
| 12 | focused library |  | 82% at 50 μM | 50 µM (Msmeg) | ||
| 2009 | [90] | HPLC reverse 4 | ||||
| 13 | focused library |  | (10) | IC50 3.5 µM | ||
| 2010 | [87] | Capillary electrophoresis | MIC | |||
| 14 | References [88,89] |  | (1) | IC50 62 µM | 3.3–6.7 µg/mL (Msmeg) 5 6.5 µg/mL (Mbov BCG) | |
| 15 | [86] |  | (2) | IC50 37 µM | 12.5 µg/mL (Msmeg) 5 50 µg/mL (Mbov BCG) | |
| 2015 | [92] | HPLC reverse 4 | MIC | |||
| 16 | virtual screening |  | (6) | nd | ||
| 17 | selection based on (6) |  | (22) | Ki 31 ± 18 μM | ||
| 18 | selection based on (22) |  | (30) | Ki 28 ± 15 μM | 9.7 µg/mL (Msmeg) | |
| 2016 | new compounds based on 2-aminothiazole scaffold (Entry 12) [86] | [93] | HPLC reverse 4 | MIC | ||
| 19 |  | (2) | IC50 12 ± 5 μM | 25 μM (Msmeg) | ||
| 20 |  | (3) | IC50 16 ± 10 μM | 25 μM (Msmeg) | ||
| 21 |  | (4) | IC50 7 ± 2 μM | 12 μM (Msmeg) | ||
| 22 |  | (5) | IC50 4 ± 1 μM | 50 μM (Msmeg) | ||
| 23 |  | (6) | IC50 18 ± 9 μM | 25 μM (Msmeg) | ||
| 24 |  | (7) | IC50 1 ± 1 μM | 12 μM (Msmeg) | ||
| 25 |  | (8) | IC50 3 ± 1 μM | 6 μM (Msmeg) | ||
| 26 |  | (9) | IC50 2 ± 1 μM | 12 μM (Msmeg) | ||
| 27 | new compounds based on triazolothiadia-zine scaffold |  | (12) | IC50 108 ± 42 μM | ||
| 28 | (Entry 18) [92] |  | (13) | IC50 19 ± 6 μM | ||
| 2016 | [94] | HPLC reverse 4 | ||||
| 29 | mechanism-based |  | (1) | 23% at 500 µM | ||
| 30 | mechanism-based |  | (2) | 24% at 500 µM | ||
| 2016 | [76] | HPLC reverse 4 | ||||
| 31 | mechanism-based |  | (46E) | 46% at 500 µM | ||
| 32 | mechanism-based |  | (46Z) | 23% at 500 µM | ||
| 2017 | [95] | HPLC reverse 4 | MIC | |||
| 33 | natural products screening |  | (4) | 100% at 500 µM | 100 µg/mL (Mtb mc2 6230) | |
| 34 | natural products screening |  | (3) | 74 ± 8% at 500 µM | >100 µg/mL (Mtb mc2 6230) | |
| 35 | natural products screening |  | (28) | 100% at 500 µM | 50 µg/mL (Mtb mc2 6230) | |
| 36 | 2017 | dynamic combinatorial chemistry | [96] | (H3+A1) | 25 µg/mL (Mtb mc2 6230) |
5.2. GlfT1 and GlfT2 Assays and Inhibitors
| Entry | Year | Target Enzyme | Structure [Reference] | Compound Id 1 | Assay Inhibitory Activity |
|---|---|---|---|---|---|
| 2004 | GlfT2 | [97] | Radiometric 2 | ||
| 1 |  | (10) | 40% at 8 mM | ||
| 2 |  | (11) | IC50 4.8 mM | ||
| 2005 | GlfT2 | [98] | Radiometric 2 | ||
| 3 |  | (25) | 72% at 8 mM | ||
| 4 |  | (3) | 85% at 8 mM | ||
| 2010 | GlfT2 | [99] | Radiometric 2 with acceptor | ||
| 5 |  | (16a) | 80% at 1 mM | ||
| 2011 | GlfT2 | [101] | Spectrophotometric 3 | ||
| 6 |  | (6c) | 75% at 4 mM | ||
| 7 |  | (6d) | 79% at 4 mM; IC50 = 332 μM | ||
| 2014 | GlfT2 | [102] | Radiometric 2 with acceptor | ||
| 8 |  | (E8) | IC50 = 0.18 mM | ||
| 2016 | GlfT2 | [103] | Spectrophotometric 3 | ||
| 9 |  | (4) | 82% at 4 mM | ||
| 10 |  | (29) | 50% at 4 mM | ||
| 2016 | GlfT1 GlfT2 | [76] | Spectrophotometric 3 | ||
| 11 |  | (46Z) | 33% at 4 mM (GlfT1)IC50 3.85 mM (GlfT2) | ||
| 12 |  | (46E) | IC50 1.09 mM (GlfT1)26% at 4 mM (GlfT2) | ||
| 2017 | GlfT2 | [104] | Spectrophotometric 3 | ||
| 13 |  | (9a) | 30% at 4 mM | ||
| 14 |  | (9b) | 100% at 4 mM; IC50 0.8mM | ||
| 15 |  | (9c) | 99% at 4 mM; IC50 0.9 mM | ||
| 16 |  | (9d) | 99% at 4 mM; IC50 1.6 mM | ||
| 17 |  | (9e) | 100% at 4 mM; IC50 2.4 mM | ||
| 2018 | GlfT2 | [105] | Spectrophotometric 3 | ||
| 18 |  | (3) | 100% at 4 mM; IC50 0.9 mM |
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Global TB Report 2019; WHO: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (accessed on 15 November 2019).
- Mdluli, K.; Kaneko, T.; Upton, A. The tuberculosis drug discovery and development pipeline and emerging drug targets. Cold Spring Harb. Perspect. Med. 2015, 5, a021154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikusova, K.; Ekins, S. Learning from the past for TB drug discovery in the future. Drug Discov. Today 2017, 22, 534–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waman, V.P.; Vedithi, S.C.; Thomas, S.E.; Bannerman, B.P.; Munir, A.; Skwark, M.J.; Malhotra, S.; Blundell, T.L. Mycobacterial genomics and structural bioinformatics: Opportunities and challenges in drug discovery. Emerg. Microbes Infect. 2019, 8, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kana, B.D.; Karakousis, P.C.; Parish, T.; Dick, T. Future target-based drug discovery for tuberculosis? Tuberculosis 2014, 94, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Tiberi, S.; du Plessis, N.; Walzl, G.; Vjecha, M.J.; Rao, M.; Ntoumi, F.; Mfinanga, S.; Kapata, N.; Mwaba, P.; McHugh, T.D.; et al. Tuberculosis: Progress and advances in development of new drugs, treatment regimens, and host-directed therapies. Lancet Infect. Dis. 2018, 18, 183–198. [Google Scholar] [CrossRef]
- Daffe, M.; Marrakchi, H. Unraveling the structure of the mycobacterial envelope. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Banerjee, A.; Dubnau, E.; Quemard, A.; Balasubramanian, V.; Um, K.S.; Wilson, T.; Collins, D.; de Lisle, G.; Jacobs, W.R., Jr. InhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 1994, 263, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Mikusova, K.; Slayden, R.A.; Besra, G.S.; Brennan, P.J. Biogenesis of the mycobacterial cell wall and the site of action of ethambutol. Antimicrob. Agents Chemother. 1995, 39, 2484–2489. [Google Scholar] [CrossRef] [Green Version]
- Pan, F.; Jackson, M.; Ma, Y.; McNeil, M. Cell wall core galactofuran synthesis is essential for growth of mycobacteria. J. Bacteriol. 2001, 183, 3991–3998. [Google Scholar] [CrossRef] [Green Version]
- Mendes, V.; Blundell, T.L. Targeting tuberculosis using structure-guided fragment-based drug design. Drug Discov. Today 2017, 22, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Crespo, R.A.; Dang, Q.; Zhou, N.E.; Guthrie, L.M.; Snavely, T.C.; Dong, W.; Loesch, K.A.; Suzuki, T.; You, L.; Wang, W.; et al. Structure-guided drug design of 6-substituted adenosine analogues as potent inhibitors of Mycobacterium tuberculosis adenosine kinase. J. Med. Chem. 2019, 62, 4483–4499. [Google Scholar] [CrossRef] [Green Version]
- Gough, G.A. The specific carbohydrate of the tubercle bacillus. Biochem. J. 1932, 26, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Haworth, N.; Kent, P.W.; Stacey, M. The constitution of a lipoid-bound polysaccharide from M. tuberculosis, human strain. J. Chem. Soc. 1948, 10, 1220–1224. [Google Scholar] [CrossRef]
- McNeil, M.R.; Brennan, P.J. Structure, function and biogenesis of the cell envelope of mycobacteria in relation to bacterial physiology, pathogenesis and drug resistance; some thoughts and possibilities arising from recent structural information. Res. Microbiol. 1991, 142, 451–463. [Google Scholar] [CrossRef]
- Misaki, A.; Yukawa, S.; Tsuchiya, K.; Yamasaki, T. Studies on cell walls of mycobacteria. I. Chemical and biological properties of the cell walls and the mucopeptide of BCG. J. Biochem. 1966, 59, 388–396. [Google Scholar] [CrossRef]
- Kanetsuna, F. Chemical analyses of mycobacterial cell walls. Biochim. Biophys. Acta 1968, 158, 130–143. [Google Scholar] [CrossRef]
- McNeil, M.; Daffe, M.; Brennan, P.J. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J. Biol. Chem. 1990, 265, 18200–18206. [Google Scholar]
- McNeil, M.; Wallner, S.J.; Hunter, S.W.; Brennan, P.J. Demonstration that the galactosyl and arabinosyl residues in the cell-wall arabinogalactan of Mycobacterium leprae and Mycobacterium tuberculosis are furanoid. Carbohydr. Res. 1987, 166, 299–308. [Google Scholar] [CrossRef]
- Daffe, M.; Brennan, P.J.; McNeil, M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. J. Biol. Chem. 1990, 265, 6734–6743. [Google Scholar]
- McNeil, M.; Daffe, M.; Brennan, P.J. Location of the mycolyl ester substituents in the cell walls of mycobacteria. J. Biol. Chem. 1991, 266, 13217–13223. [Google Scholar]
- Besra, G.S.; Khoo, K.H.; McNeil, M.R.; Dell, A.; Morris, H.R.; Brennan, P.J. A new interpretation of the structure of the mycolyl-arabinogalactan complex of Mycobacterium tuberculosis as revealed through characterization of oligoglycosylalditol fragments by fast-atom bombardment mass spectrometry and 1H nuclear magnetic resonance spectroscopy. Biochemistry 1995, 34, 4257–4266. [Google Scholar] [CrossRef]
- Bhamidi, S.; Scherman, M.S.; Rithner, C.D.; Prenni, J.E.; Chatterjee, D.; Khoo, K.H.; McNeil, M.R. The identification and location of succinyl residues and the characterization of the interior arabinan region allow for a model of the complete primary structure of Mycobacterium tuberculosis mycolyl arabinogalactan. J. Biol. Chem. 2008, 283, 12992–13000. [Google Scholar] [CrossRef] [Green Version]
- Bhamidi, S.; Scherman, M.S.; Jones, V.; Crick, D.C.; Belisle, J.T.; Brennan, P.J.; McNeil, M.R. Detailed structural and quantitative analysis reveals the spatial organization of the cell walls of in vivo grown Mycobacterium leprae and in vitro grown Mycobacterium tuberculosis. J. Biol. Chem. 2011, 286, 23168–23177. [Google Scholar] [CrossRef] [Green Version]
- Skovierova, H.; Larrouy-Maumus, G.; Pham, H.; Belanova, M.; Barilone, N.; Dasgupta, A.; Mikusova, K.; Gicquel, B.; Gilleron, M.; Brennan, P.J.; et al. Biosynthetic origin of the galactosamine substituent of arabinogalactan in Mycobacterium tuberculosis. J. Biol. Chem. 2010, 285, 41348–41355. [Google Scholar] [CrossRef] [Green Version]
- Palcekova, Z.; Angala, S.K.; Belardinelli, J.M.; Eskandarian, H.A.; Joe, M.; Brunton, R.; Rithner, C.; Jones, V.; Nigou, J.; Lowary, T.L.; et al. Disruption of the SucT acyltransferase in Mycobacterium smegmatis abrogates succinylation of cell envelope polysaccharides. J. Biol. Chem. 2019, 294, 10325–10335. [Google Scholar] [CrossRef]
- Vilcheze, C.; Kremer, L. Acid-fast positive and acid-fast negative Mycobacterium tuberculosis: The Koch paradox. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Dulberger, C.L.; Rubin, E.J.; Boutte, C.C. The mycobacterial cell envelope—A moving target. Nat. Rev. Microbiol. 2020, 18, 47–59. [Google Scholar] [CrossRef]
- Hayashi, J.M.; Richardson, K.; Melzer, E.S.; Sandler, S.J.; Aldridge, B.B.; Siegrist, M.S.; Morita, Y.S. Stress-induced reorganization of the mycobacterial membrane domain. mBio 2018, 9, e01823-17. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, C.; Leis, A.; Niederweis, M.; Plitzko, J.M.; Engelhardt, H. Disclosure of the mycobacterial outer membrane: Cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. USA 2008, 105, 3963–3967. [Google Scholar] [CrossRef] [Green Version]
- Zuber, B.; Chami, M.; Houssin, C.; Dubochet, J.; Griffiths, G.; Daffe, M. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 2008, 190, 5672–5680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araki, Y.; Ito, E. Linkage units in cell walls of gram-positive bacteria. Crit. Rev. Microbiol. 1989, 17, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Snapper, S.B.; Melton, R.E.; Mustafa, S.; Kieser, T.; Jacobs, W.R., Jr. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 1990, 4, 1911–1919. [Google Scholar] [CrossRef]
- Pelicic, V.; Jackson, M.; Reyrat, J.M.; Jacobs, W.R., Jr.; Gicquel, B.; Guilhot, C. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 1997, 94, 10955–10960. [Google Scholar] [CrossRef] [Green Version]
- Triccas, J.A.; Parish, T.; Britton, W.J.; Gicquel, B. An inducible expression system permitting the efficient purification of a recombinant antigen from Mycobacterium smegmatis. FEMS Microbiol. Lett. 1998, 167, 151–156. [Google Scholar] [CrossRef]
- Mikusova, K.; Mikus, M.; Besra, G.S.; Hancock, I.; Brennan, P.J. Biosynthesis of the linkage region of the mycobacterial cell wall. J. Biol. Chem. 1996, 271, 7820–7828. [Google Scholar] [CrossRef] [Green Version]
- Takayama, K.; Goldman, D.S. Enzymatic synthesis of mannosyl-1-phosphoryl-decaprenol by a cell-free system of Mycobacterium tuberculosis. J. Biol. Chem. 1970, 245, 6251–6257. [Google Scholar]
- Wolucka, B.A.; McNeil, M.R.; de Hoffmann, E.; Chojnacki, T.; Brennan, P.J. Recognition of the lipid intermediate for arabinogalactan/arabinomannan biosynthesis and its relation to the mode of action of ethambutol on mycobacteria. J. Biol. Chem. 1994, 269, 23328–23335. [Google Scholar]
- Ward, J.B. Tunicamycin inhibition of bacterial wall polymer synthesis. FEBS Lett. 1977, 78, 151–154. [Google Scholar] [CrossRef] [Green Version]
- Mikusova, K.; Yagi, T.; Stern, R.; McNeil, M.R.; Besra, G.S.; Crick, D.C.; Brennan, P.J. Biosynthesis of the galactan component of the mycobacterial cell wall. J. Biol. Chem. 2000, 275, 33890–33897. [Google Scholar] [CrossRef] [Green Version]
- Nassau, P.M.; Martin, S.L.; Brown, R.E.; Weston, A.; Monsey, D.; McNeil, M.R.; Duncan, K. Galactofuranose biosynthesis in Escherichia coli K-12: Identification and cloning of UDP-galactopyranose mutase. J. Bacteriol. 1996, 178, 1047–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weston, A.; Stern, R.J.; Lee, R.E.; Nassau, P.M.; Monsey, D.; Martin, S.L.; Scherman, M.S.; Besra, G.S.; Duncan, K.; McNeil, M.R. Biosynthetic origin of mycobacterial cell wall galactofuranosyl residues. Tuber. Lung. Dis. 1997, 78, 123–131. [Google Scholar] [CrossRef]
- Belanova, M.; Dianiskova, P.; Brennan, P.J.; Completo, G.C.; Rose, N.L.; Lowary, T.L.; Mikusova, K. Galactosyl transferases in mycobacterial cell wall synthesis. J. Bacteriol. 2008, 190, 1141–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, G.; Neal, B.; Liu, D.; Hobbs, M.; Packer, N.H.; Batley, M.; Redmond, J.W.; Lindquist, L.; Reeves, P. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J. Bacteriol. 1994, 176, 4144–4156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belanger, A.E.; Inamine, J.M. Genetics of cell wall biosythesis. In Molecular Genetics of Mycobacteria; Hatfull, G.F., Jacobs, W.R., Jr., Eds.; ASM Press: Washington, DC, USA, 2000; pp. 191–202. [Google Scholar]
- Sanders, D.A.; Staines, A.G.; McMahon, S.A.; McNeil, M.R.; Whitfield, C.; Naismith, J.H. UDP-galactopyranose mutase has a novel structure and mechanism. Nat. Struct. Biol. 2001, 8, 858–863. [Google Scholar] [CrossRef]
- Richards, M.R.; Lowary, T.L. Chemistry and biology of galactofuranose-containing polysaccharides. ChemBioChem 2009, 10, 1920–1938. [Google Scholar] [CrossRef]
- Soltero-Higgin, M.; Carlson, E.E.; Gruber, T.D.; Kiessling, L.L. A unique catalytic mechanism for UDP-galactopyranose mutase. Nat. Struct. Mol. Biol. 2004, 11, 539–543. [Google Scholar] [CrossRef]
- Sun, H.G.; Ruszczycky, M.W.; Chang, W.C.; Thibodeaux, C.J.; Liu, H.W. Nucleophilic participation of reduced flavin coenzyme in mechanism of UDP-galactopyranose mutase. J. Biol. Chem. 2012, 287, 4602–4608. [Google Scholar] [CrossRef] [Green Version]
- Tanner, J.J.; Boechi, L.; Andrew McCammon, J.; Sobrado, P. Structure, mechanism, and dynamics of UDP-galactopyranose mutase. Arch. Biochem. Biophys. 2014, 544, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Beis, K.; Srikannathasan, V.; Liu, H.; Fullerton, S.W.; Bamford, V.A.; Sanders, D.A.; Whitfield, C.; McNeil, M.R.; Naismith, J.H. Crystal structures of Mycobacteria tuberculosis and Klebsiella pneumoniae UDP-galactopyranose mutase in the oxidised state and Klebsiella pneumoniae UDP-galactopyranose mutase in the (active) reduced state. J. Mol. Biol. 2005, 348, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Van Straaten, K.E.; Kuttiyatveetil, J.R.; Sevrain, C.M.; Villaume, S.A.; Jimenez-Barbero, J.; Linclau, B.; Vincent, S.P.; Sanders, D.A. Structural basis of ligand binding to UDP-galactopyranose mutase from Mycobacterium tuberculosis using substrate and tetrafluorinated substrate analogues. J. Am. Chem. Soc. 2015, 137, 1230–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breton, C.; Bettler, E.; Joziasse, D.H.; Geremia, R.A.; Imberty, A. Sequence-function relationships of prokaryotic and eukaryotic galactosyltransferases. J. Biochem. 1998, 123, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Saxena, I.M.; Brown, R.M., Jr.; Fevre, M.; Geremia, R.A.; Henrissat, B. Multidomain architecture of beta-glycosyl transferases: Implications for mechanism of action. J. Bacteriol. 1995, 177, 1419–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kremer, L.; Dover, L.G.; Morehouse, C.; Hitchin, P.; Everett, M.; Morris, H.R.; Dell, A.; Brennan, P.J.; McNeil, M.R.; Flaherty, C.; et al. Galactan biosynthesis in Mycobacterium tuberculosis. Identification of a bifunctional UDP-galactofuranosyltransferase. J. Biol. Chem. 2001, 276, 26430–26440. [Google Scholar] [CrossRef] [Green Version]
- Rose, N.L.; Completo, G.C.; Lin, S.J.; McNeil, M.; Palcic, M.M.; Lowary, T.L. Expression, purification, and characterization of a galactofuranosyltransferase involved in Mycobacterium tuberculosis arabinogalactan biosynthesis. J. Am. Chem. Soc. 2006, 128, 6721–6729. [Google Scholar] [CrossRef]
- Splain, R.A.; Kiessling, L.L. Synthesis of galactofuranose-based acceptor substrates for the study of the carbohydrate polymerase GlfT2. Bioorg. Med. Chem. 2010, 18, 3753–3759. [Google Scholar] [CrossRef] [Green Version]
- May, J.F.; Splain, R.A.; Brotschi, C.; Kiessling, L.L. A tethering mechanism for length control in a processive carbohydrate polymerization. Proc. Natl. Acad. Sci. USA 2009, 106, 11851–11856. [Google Scholar] [CrossRef] [Green Version]
- Levengood, M.R.; Splain, R.A.; Kiessling, L.L. Monitoring processivity and length control of a carbohydrate polymerase. J. Am. Chem. Soc. 2011, 133, 12758–12766. [Google Scholar] [CrossRef] [Green Version]
- Szczepina, M.G.; Zheng, R.B.; Completo, G.C.; Lowary, T.L.; Pinto, B.M. STD-NMR studies suggest that two acceptor substrates for GlfT2, a bifunctional galactofuranosyltransferase required for the biosynthesis of Mycobacterium tuberculosis arabinogalactan, compete for the same binding site. ChemBioChem 2009, 10, 2052–2059. [Google Scholar] [CrossRef]
- May, J.F.; Levengood, M.R.; Splain, R.A.; Brown, C.D.; Kiessling, L.L. A processive carbohydrate polymerase that mediates bifunctional catalysis using a single active site. Biochemistry 2012, 51, 1148–1159. [Google Scholar] [CrossRef] [Green Version]
- Wheatley, R.W.; Zheng, R.B.; Richards, M.R.; Lowary, T.L.; Ng, K.K. Tetrameric structure of the GlfT2 galactofuranosyltransferase reveals a scaffold for the assembly of mycobacterial arabinogalactan. J. Biol. Chem. 2012, 287, 28132–28143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janos, P.; Kozmon, S.; Tvaroska, I.; Koca, J. How Mycobacterium tuberculosis galactofuranosyl transferase 2 (GlfT2) generates alternating β-(1-6) and β-(1-5) linkages: A QM/MM molecular dynamics study of the chemical steps. Chemistry 2018, 24, 7051–7059. [Google Scholar] [CrossRef] [PubMed]
- Wesener, D.A.; Levengood, M.R.; Kiessling, L.L. Comparing galactan biosynthesis in Mycobacterium tuberculosis and Corynebacterium diphtheriae. J. Biol. Chem. 2017, 292, 2944–2955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alderwick, L.J.; Radmacher, E.; Seidel, M.; Gande, R.; Hitchen, P.G.; Morris, H.R.; Dell, A.; Sahm, H.; Eggeling, L.; Besra, G.S. Deletion of Cg-emb in corynebacterianeae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J. Biol. Chem. 2005, 280, 32362–32371. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Zheng, R.B.; Koizumi, A.; Han, L.; Klassen, J.S.; Lowary, T.L. Synthetic polyprenol-pyrophosphate linked oligosaccharides are efficient substrates for mycobacterial galactan biosynthetic enzymes. Org. Biomol. Chem. 2018, 16, 1939–1957. [Google Scholar] [CrossRef]
- Mikusova, K.; Belanova, M.; Kordulakova, J.; Honda, K.; McNeil, M.R.; Mahapatra, S.; Crick, D.C.; Brennan, P.J. Identification of a novel galactosyl transferase involved in biosynthesis of the mycobacterial cell wall. J. Bacteriol. 2006, 188, 6592–6598. [Google Scholar] [CrossRef] [Green Version]
- Peltier, P.; Belanova, M.; Dianiskova, P.; Zhou, R.; Zheng, R.B.; Pearcey, J.A.; Joe, M.; Brennan, P.J.; Nugier-Chauvin, C.; Ferrieres, V.; et al. Synthetic UDP-furanoses as potent inhibitors of mycobacterial galactan biogenesis. Chem. Biol. 2010, 17, 1356–1366. [Google Scholar] [CrossRef]
- Brown, C.D.; Rusek, M.S.; Kiessling, L.L. Fluorosugar chain termination agents as probes of the sequence specificity of a carbohydrate polymerase. J. Am. Chem. Soc. 2012, 134, 6552–6555. [Google Scholar] [CrossRef] [Green Version]
- Alderwick, L.J.; Dover, L.G.; Veerapen, N.; Gurcha, S.S.; Kremer, L.; Roper, D.L.; Pathak, A.K.; Reynolds, R.C.; Besra, G.S. Expression, purification and characterisation of soluble GlfT and the identification of a novel galactofuranosyltransferase Rv3782 involved in priming GlfT-mediated galactan polymerisation in Mycobacterium tuberculosis. Protein Expr. Purif. 2008, 58, 332–341. [Google Scholar] [CrossRef]
- Martinez Farias, M.A.; Kincaid, V.A.; Annamalai, V.R.; Kiessling, L.L. Isoprenoid phosphonophosphates as glycosyltransferase acceptor substrates. J. Am. Chem. Soc. 2014, 136, 8492–8495. [Google Scholar] [CrossRef] [Green Version]
- DeJesus, M.A.; Gerrick, E.R.; Xu, W.; Park, S.W.; Long, J.E.; Boutte, C.C.; Rubin, E.J.; Schnappinger, D.; Ehrt, S.; Fortune, S.M.; et al. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 2017, 8, e02133-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltier, P.; Euzen, R.; Daniellou, R.; Nugier-Chauvin, C.; Ferrieres, V. Recent knowledge and innovations related to hexofuranosides: Structure, synthesis and applications. Carbohydr. Res. 2008, 343, 1897–1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tefsen, B.; Ram, A.F.; van Die, I.; Routier, F.H. Galactofuranose in eukaryotes: Aspects of biosynthesis and functional impact. Glycobiology 2012, 22, 456–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederic, C.J.; Tikad, A.; Fu, J.; Pan, W.; Zheng, R.B.; Koizumi, A.; Xue, X.; Lowary, T.L.; Vincent, S.P. Synthesis of unprecedented sulfonylated phosphono-exo-glycals designed as inhibitors of the three mycobacterial galactofuranose processing enzymes. Chemistry 2016, 22, 15913–15920. [Google Scholar] [CrossRef] [PubMed]
- Errey, J.C.; Mukhopadhyay, B.; Kartha, K.P.; Field, R.A. Flexible enzymatic and chemo-enzymatic approaches to a broad range of uridine-diphospho-sugars. Chem. Commun. 2004, 2706–2707. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Monsey, D.; Weston, A.; Duncan, K.; Rithner, C.; McNeil, M. Enzymatic synthesis of UDP-galactofuranose and an assay for UDP-galactopyranose mutase based on high-performance liquid chromatography. Anal. Biochem. 1996, 242, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.E.; Smith, M.D.; Nash, R.J.; Griffiths, R.C.; McNeil, M.; Grewal, R.K.; Yan, W.; Besra, G.S.; Brennan, P.J.; Fleet, G.W. Inhibition of UDP-Gal mutase and mycobacterial galactan biosynthesis by pyrrolidine analogues of galactofuranose. Tetrahedron Lett. 1997, 38, 6733–6736. [Google Scholar] [CrossRef]
- Scherman, M.S.; Winans, K.A.; Stern, R.J.; Jones, V.; Bertozzi, C.R.; McNeil, M.R. Drug targeting Mycobacterium tuberculosis cell wall synthesis: Development of a microtiter plate-based screen for UDP-galactopyranose mutase and identification of an inhibitor from a uridine-based library. Antimicrob. Agents Chemother. 2003, 47, 378–382. [Google Scholar] [CrossRef] [Green Version]
- Tangallapally, R.P.; Yendapally, R.; Lee, R.E.; Hevener, K.; Jones, V.C.; Lenaerts, A.J.; McNeil, M.R.; Wang, Y.; Franzblau, S.; Lee, R.E. Synthesis and evaluation of nitrofuranylamides as novel antituberculosis agents. J. Med. Chem. 2004, 47, 5276–5283. [Google Scholar] [CrossRef]
- Helm, J.S.; Hu, Y.; Chen, L.; Gross, B.; Walker, S. Identification of active-site inhibitors of MurG using a generalizable, high-throughput glycosyltransferase screen. J. Am. Chem. Soc. 2003, 125, 11168–11169. [Google Scholar] [CrossRef]
- Soltero-Higgin, M.; Carlson, E.E.; Phillips, J.H.; Kiessling, L.L. Identification of inhibitors for UDP-galactopyranose mutase. J. Am. Chem. Soc. 2004, 126, 10532-3. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.E.; May, J.F.; Kiessling, L.L. Chemical probes of UDP-galactopyranose mutase. Chem. Biol. 2006, 13, 825–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Dykhuizen, E.C.; May, J.F.; Tongpenyai, A.; Kiessling, L.L. Inhibitors of UDP-galactopyranose mutase thwart mycobacterial growth. J. Am. Chem. Soc. 2008, 130, 6706–6707. [Google Scholar] [CrossRef]
- Borrelli, S.; Zandberg, W.F.; Mohan, S.; Ko, M.; Martinez-Gutierrez, F.; Partha, S.K.; Sanders, D.A.; Av-Gay, Y.; Pinto, B.M. Antimycobacterial activity of UDP-galactopyranose mutase inhibitors. Int. J. Antimicrob. Agents 2010, 36, 364–368. [Google Scholar] [CrossRef]
- Castagnolo, D.; De Logu, A.; Radi, M.; Bechi, B.; Manetti, F.; Magnani, M.; Supino, S.; Meleddu, R.; Chisu, L.; Botta, M. Synthesis, biological evaluation and SAR study of novel pyrazole analogues as inhibitors of Mycobacterium tuberculosis. Bioorg. Med. Chem. 2008, 16, 8587–8591. [Google Scholar] [CrossRef] [Green Version]
- Manetti, F.; Magnani, M.; Castagnolo, D.; Passalacqua, L.; Botta, M.; Corelli, F.; Saddi, M.; Deidda, D.; De Logu, A. Ligand-based virtual screening, parallel solution-phase and microwave-assisted synthesis as tools to identify and synthesize new inhibitors of Mycobacterium tuberculosis. ChemMedChem 2006, 1, 973–989. [Google Scholar] [CrossRef]
- Dykhuizen, E.C.; Kiessling, L.L. Potent ligands for prokaryotic UDP-galactopyranose mutase that exploit an enzyme subsite. Org. Lett. 2009, 11, 193–196. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Colombo, C.; Kuttiyatveetil, J.R.; Zalatar, N.; van Straaten, K.E.; Mohan, S.; Sanders, D.A.; Pinto, B.M. A second, druggable binding site in UDP-galactopyranose mutase from Mycobacterium tuberculosis? ChemBioChem 2016, 17, 2264–2273. [Google Scholar] [CrossRef]
- Kincaid, V.A.; London, N.; Wangkanont, K.; Wesener, D.A.; Marcus, S.A.; Heroux, A.; Nedyalkova, L.; Talaat, A.M.; Forest, K.T.; Shoichet, B.K.; et al. Virtual screening for UDP-galactopyranose mutase ligands identifies a new class of antimycobacterial agents. ACS Chem. Biol. 2015, 10, 2209–2218. [Google Scholar] [CrossRef] [Green Version]
- Winton, V.J.; Aldrich, C.; Kiessling, L.L. Carboxylate surrogates enhance the antimycobacterial activity of UDP-galactopyranose mutase probes. ACS Infect. Dis. 2016, 2, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi-Amiri, Y.; Mohan, S.; Borrelli, S.; Slowski, K.; Sanders, D.A.; Pinto, B.M. Mechanism-based candidate inhibitors of uridine diphosphate galactopyranose mutase (UGM). Carbohydr. Res. 2016, 419, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Villaume, S.A.; Fu, J.; N’Go, I.; Liang, H.; Lou, H.; Kremer, L.; Pan, W.; Vincent, S.P. Natural and synthetic flavonoids as potent Mycobacterium tuberculosis UGM inhibitors. Chemistry 2017, 23, 10423–10429. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Fu, H.; Dieu, M.; Halloum, I.; Kremer, L.; Xia, Y.; Pan, W.; Vincent, S.P. Identification of inhibitors targeting Mycobacterium tuberculosis cell wall biosynthesis via dynamic combinatorial chemistry. Chem. Commun. 2017, 53, 10632–10635. [Google Scholar] [CrossRef]
- Cren, S.; Gurcha, S.S.; Blake, A.J.; Besra, G.S.; Thomas, N.R. Synthesis and biological evaluation of new inhibitors of UDP-Galf transferase—A key enzyme in M. tuberculosis cell wall biosynthesis. Org. Biomol. Chem. 2004, 2, 2418–2420. [Google Scholar] [CrossRef] [PubMed]
- Cren, S.; Wilson, C.; Thomas, N.R. A rapid synthesis of hexofuranose-like iminosugars using ring-closing metathesis. Org. Lett. 2005, 7, 3521–3523. [Google Scholar] [CrossRef]
- Trunkfield, A.E.; Gurcha, S.S.; Besra, G.S.; Bugg, T.D. Inhibition of Escherichia coli glycosyltransferase MurG and Mycobacterium tuberculosis Gal transferase by uridine-linked transition state mimics. Bioorg. Med. Chem. 2010, 18, 2651–2663. [Google Scholar] [CrossRef] [Green Version]
- Rose, N.L.; Zheng, R.B.; Pearcey, J.; Zhou, R.; Completo, G.C.; Lowary, T.L. Development of a coupled spectrophotometric assay for GlfT2, a bifunctional mycobacterial galactofuranosyltransferase. Carbohydr. Res. 2008, 343, 2130–2139. [Google Scholar] [CrossRef]
- Vembaiyan, K.; Pearcey, J.A.; Bhasin, M.; Lowary, T.L.; Zou, W. Synthesis of sugar-amino acid-nucleosides as potential glycosyltransferase inhibitors. Bioorg. Med. Chem. 2011, 19, 58–66. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Eppe, G.; Tikad, A.; Pan, W.; El Bkassiny, S.; Gurcha, S.S.; Arda, A.; Jimenez-Barbero, J.; Besra, G.S.; Vincent, S.P. Selectfluor and NFSI exo-glycal fluorination strategies applied to the enhancement of the binding affinity of galactofuranosyltransferase GlfT2 inhibitors. Chemistry 2014, 20, 15208–15215. [Google Scholar] [CrossRef]
- Lowary, T.L.; Li, J. Synthesis and evaluation of bicyclo[3.1.0]hexane-based UDP-Galf analogues as inhibitors of the mycobacterial galactofuranosyltransferase GlfT2. Molecules 2016, 21, 1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocaud, C.; Maujoin, A.; Zheng, R.B.; Lowary, T.L.; Rodrigues, N.; Percina, N.; Chartier, A.; Buron, F.; Routier, S.; Nicolas, C.; et al. Triazole-linked iminosugars and aromatic systems as simplified UDP-Galf mimics: Synthesis and preliminary evaluation as Galf-transferase inhibitors. Eur. J. Org. Chem. 2017, 2017, 6192–6201. [Google Scholar] [CrossRef]
- Cocaud, C.; Zheng, R.B.; Lowary, T.L.; Poisson, T.; Pannecoucke, X.; Nicolas, C.; Martin, O.R. 1-C-phosphonomethyl- and 1-C-difluorophosphonomethyl-1,4-imino-l-arabinitols as Galf transferase inhibitors: A comparison. Carbohydr. Res. 2018, 461, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Ortiz CL, D.; Completo, G.C.; Nacario, R.C.; Nellas, R.B. Potential inhibitors of galactofuranosyltransferase 2 (GlfT2): Molecular docking, 3D-QSAR, and in silico ADMETox Studies. Sci. Rep. 2019, 9, 17096. [Google Scholar] [CrossRef] [PubMed]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Sala, C.; Hartkoorn, R.C. Tuberculosis drugs: New candidates and how to find more. Future Microbiol. 2011, 6, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; LaVerriere, E.; Office, E.; Stanley, M.; Meyer, E.; Kawate, T.; Gomez, J.E.; Audette, R.E.; Bandyopadhyay, N.; Betancourt, N.; et al. Large-scale chemical-genetics yields new M. tuberculosis inhibitor classes. Nature 2019, 571, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Melief, E.; Kokoczka, R.; Files, M.; Bailey, M.A.; Alling, T.; Li, H.; Ahn, J.; Misquith, A.; Korkegian, A.; Roberts, D.; et al. Construction of an overexpression library for Mycobacterium tuberculosis. Biol. Methods Protoc. 2018, 3, bpy009. [Google Scholar] [CrossRef]
- Singh, V.; Mizrahi, V. Identification and validation of novel drug targets in Mycobacterium tuberculosis. Drug Discov. Today 2017, 22, 503–509. [Google Scholar] [CrossRef]
- Evans, J.C.; Mizrahi, V. The application of tetracyclineregulated gene expression systems in the validation of novel drug targets in Mycobacterium tuberculosis. Front. Microbiol. 2015, 6, 812. [Google Scholar] [CrossRef] [Green Version]
- Rock, J.M.; Hopkins, F.F.; Chavez, A.; Diallo, M.; Chase, M.R.; Gerrick, E.R.; Pritchard, J.R.; Church, G.M.; Rubin, E.J.; Sassetti, C.M.; et al. Programmable transcriptional repression in mycobacteria using an orthogonal CRISPR interference platform. Nat. Microbiol. 2017, 2, 16274. [Google Scholar] [CrossRef] [PubMed]
- Meniche, X.; Otten, R.; Siegrist, M.S.; Baer, C.E.; Murphy, K.C.; Bertozzi, C.R.; Sassetti, C.M. Subpolar addition of new cell wall is directed by DivIVA in mycobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 3243–3251. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konyariková, Z.; Savková, K.; Kozmon, S.; Mikušová, K. Biosynthesis of Galactan in Mycobacterium tuberculosis as a Viable TB Drug Target? Antibiotics 2020, 9, 20. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010020
Konyariková Z, Savková K, Kozmon S, Mikušová K. Biosynthesis of Galactan in Mycobacterium tuberculosis as a Viable TB Drug Target? Antibiotics. 2020; 9(1):20. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010020
Chicago/Turabian StyleKonyariková, Zuzana, Karin Savková, Stanislav Kozmon, and Katarína Mikušová. 2020. "Biosynthesis of Galactan in Mycobacterium tuberculosis as a Viable TB Drug Target?" Antibiotics 9, no. 1: 20. https://0-doi-org.brum.beds.ac.uk/10.3390/antibiotics9010020




